| Clin Mol Hepatol > Volume 30(3); 2024 > Article |
|
ABSTRACT
Background/Aims
Methods
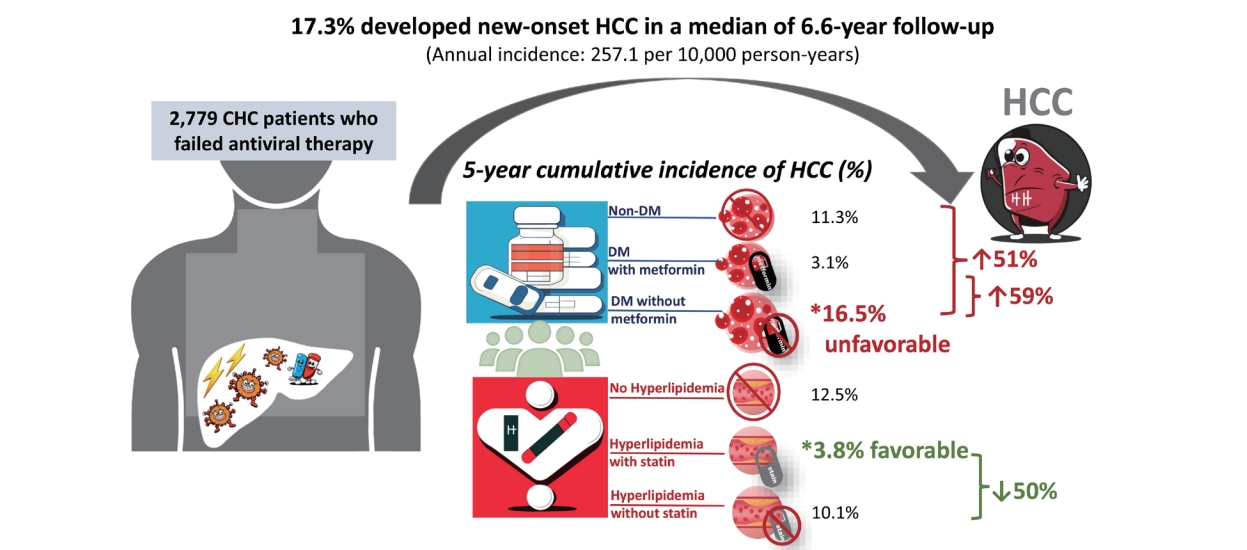
Results
ACKNOWLEDGMENTS
FOOTNOTES
SUPPLEMENTAL MATERIAL
Supplementary Figure 1.
Supplementary Figure 2.
Supplementary Figure 3.
Supplementary Figure 4.
Supplementary Figure 5.
Supplementary Table 1.
Supplementary Table 2.
Figure 1.
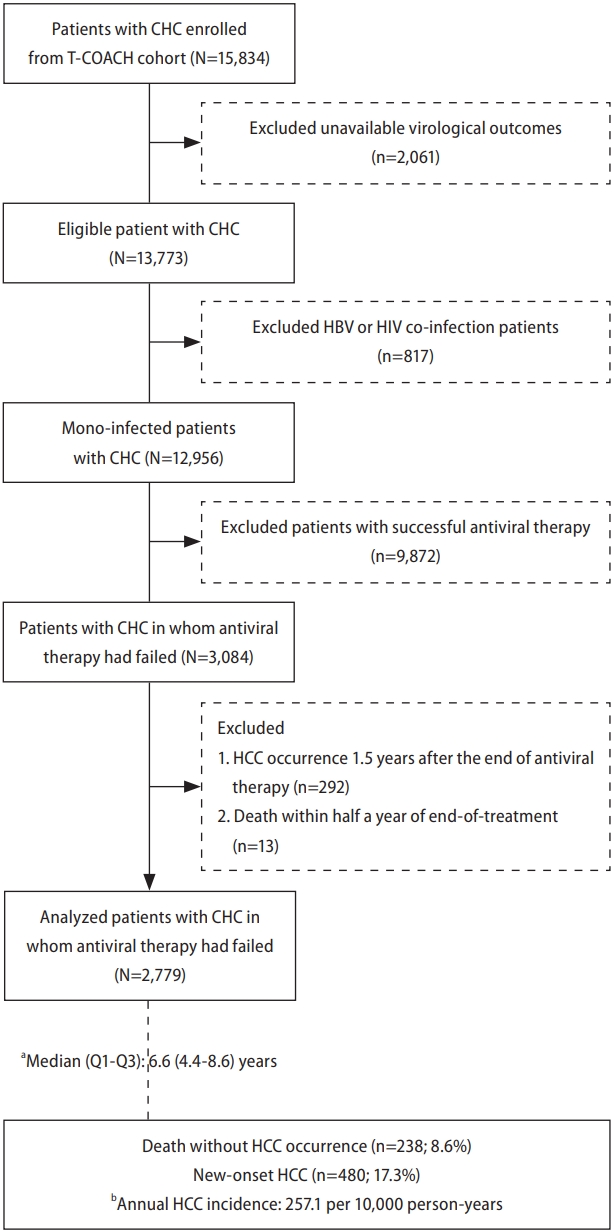
Figure 2.
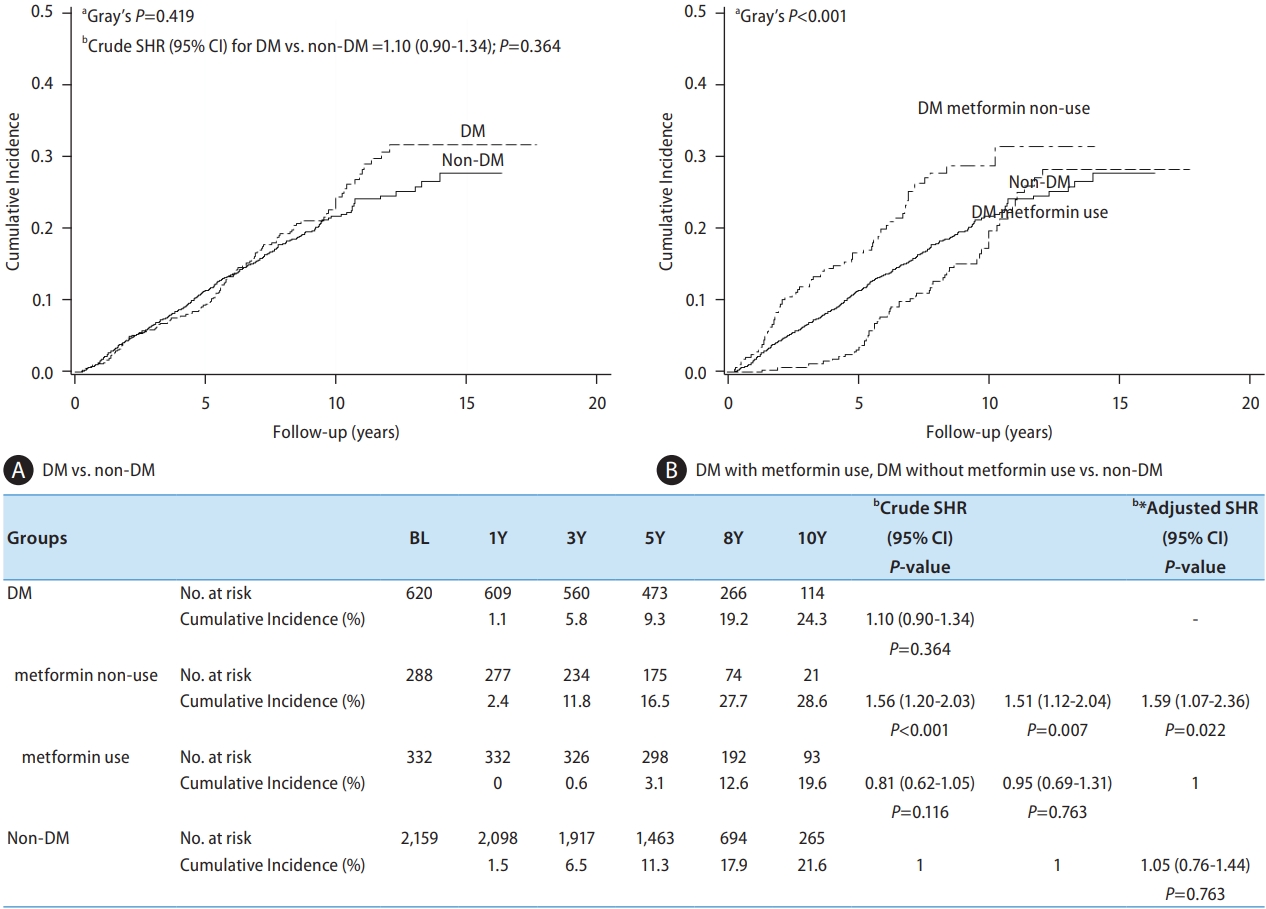
Figure 3.
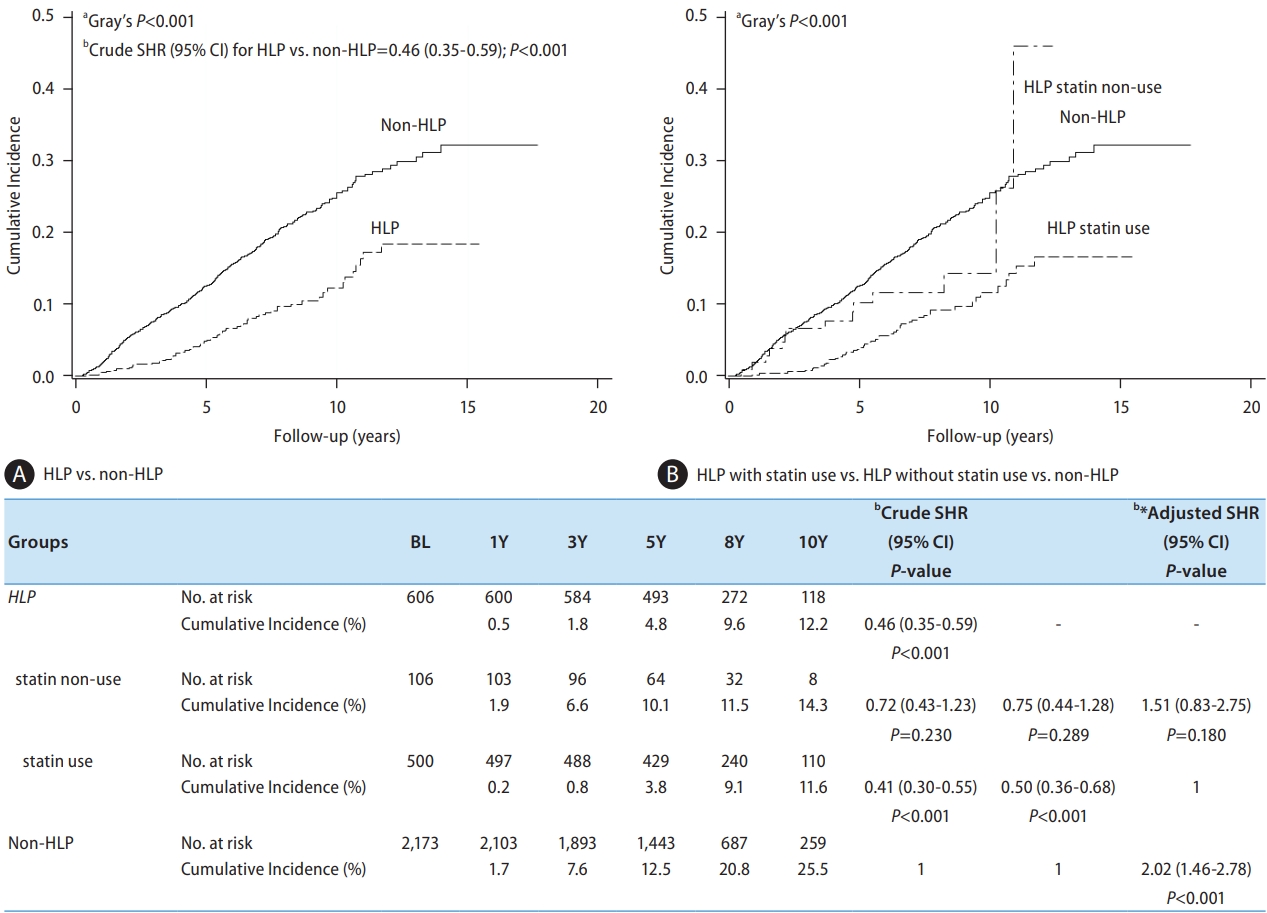
Figure 4.
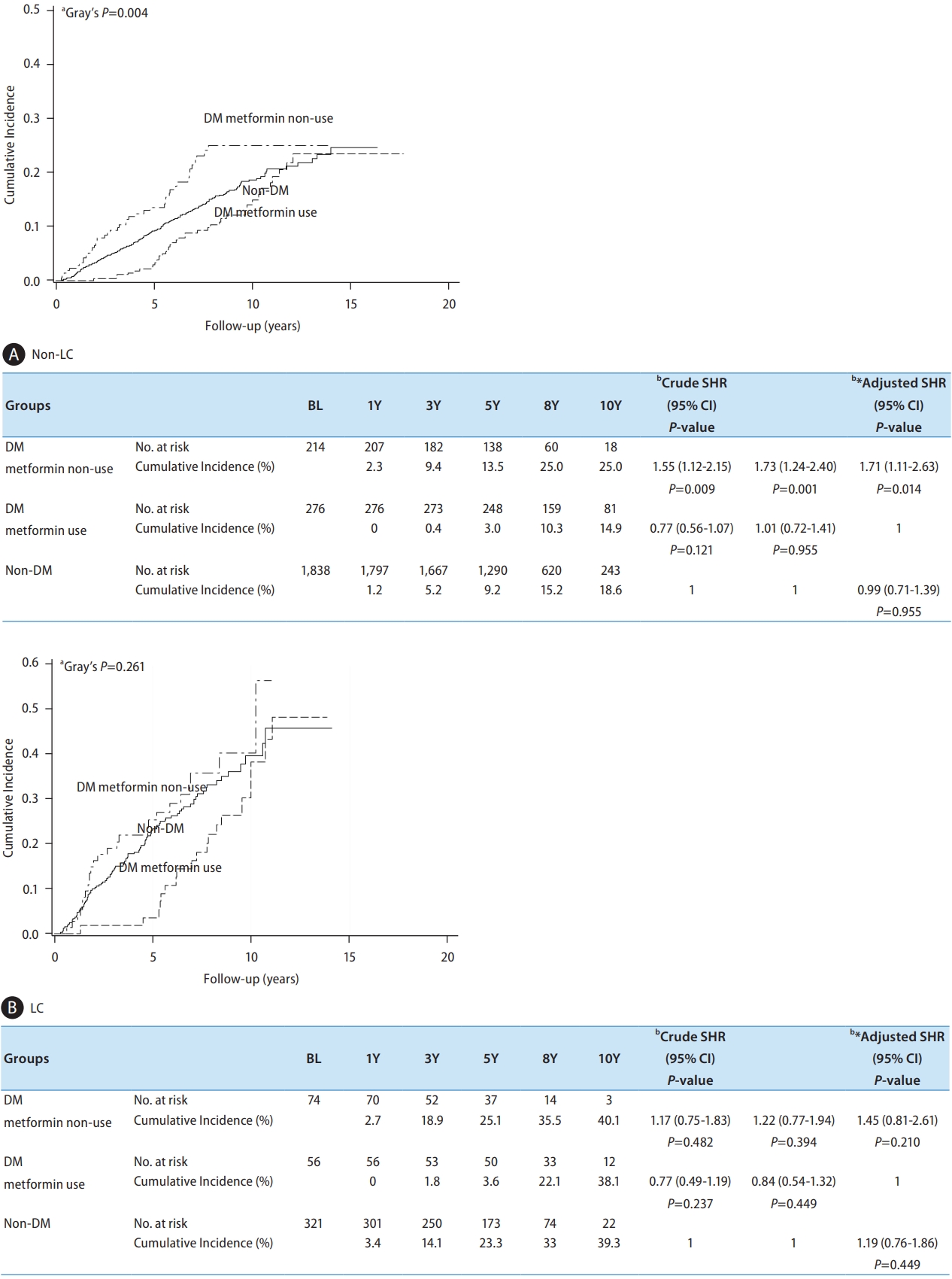
Figure 5.
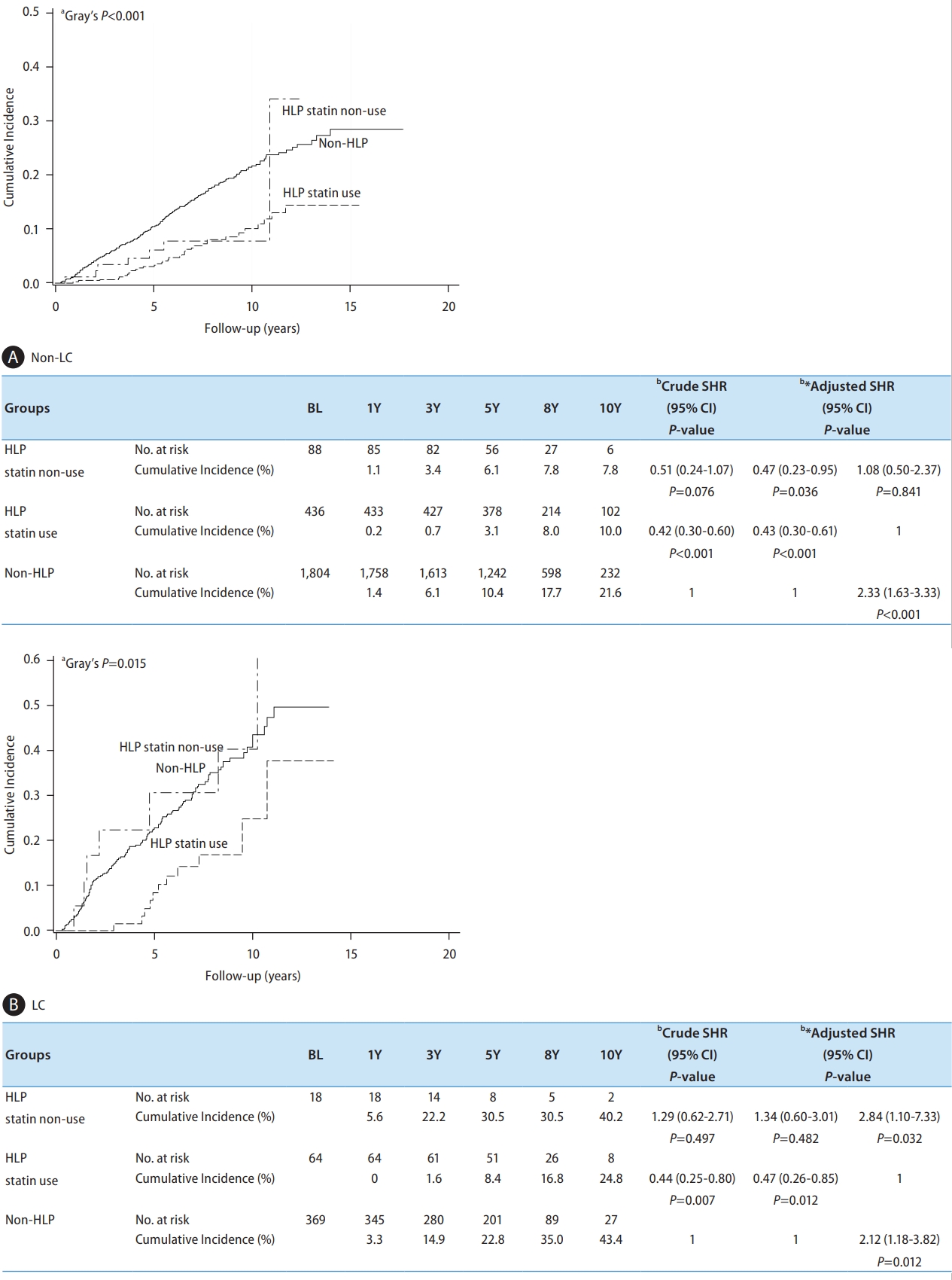
Table 1.
Values are presented as mean±standard deviation (SD) or number (%).
CHC, chronic hepatitis C; BMI, body mass index; DM, diabetes mellitus; HTN, hypertension; HLP, hyperlipidemia; AST, aspartate aminotransferase; ALT, alanine aminotransferase; FIB-4, fibrosis index based on four factors; eGFR, estimated glomerular filtration rate; HCC, hepatocellular carcinoma.
Table 2.
| Variables | Levels | No. | New-Onset | Competing |
Crude |
Adjusted |
Adjusted |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| SHR (95% CI) | P-value | SHR (95% CI) | P-value | SHR (95% CI) | P-value | |||||
| Age (years) | <65 | 2,226 | 335 (15.1) | 156 (7.0) | 1 | 1 | 1 | |||
| ≥65 | 553 | 145 (26.2) | 82 (14.8) | 1.93 (1.59–2.35) | <0.001* | 1.89 (1.52–2.36) | <0.001* | 1.89 (1.52–2.36) | <0.001* | |
| Gender | Male | 1,315 | 206 (15.7) | 139 (10.6) | 1 | 1 | 1 | |||
| Female | 1,464 | 274 (18.7) | 99 (6.8) | 1.23 (1.03–1.47) | 0.025* | 1.14 (0.92–1.40) | 0.225 | 1.14 (0.92–1.40) | 0.225 | |
| BMI (kg/m2) | <27 | 2,291 | 390 (17.0) | 186 (8.1) | 1 | - | - | |||
| ≥27 | 488 | 90 (18.4) | 52 (10.7) | 1.11 (0.88–1.39) | 0.378 | - | - | |||
| Aspirin | No | 2,490 | 435 (17.5) | 213 (8.6) | 1 | 1 | 1 | |||
| Yes | 289 | 45 (15.6) | 25 (8.7) | 0.68 (0.50–0.91) | 0.009* | 0.71 (0.51–1.00) | 0.049* | 0.71 (0.51–1.00) | 0.049* | |
| DM/Metformin | Non-DM | 2,159 | 355 (16.4) | 173 (8.0) | 1 | 1 | 1.05 (0.76–1.44) | 0.763 | ||
| DM/metformin (+) | 332 | 57 (17.2) | 25 (7.5) | 0.81 (0.62–1.05) | 0.116 | 0.95 (0.69–1.31) | 0.763 | 1 | ||
| DM/metformin (-) | 288 | 68 (23.6) | 40 (13.9) | 1.56 (1.20–2.03) | <0.001* | 1.51 (1.12–2.04) | 0.007* | 1.59 (1.07–2.36) | 0.022* | |
| HLP/Statin | Non-HLP | 2,173 | 419 (19.3) | 207 (9.5) | 1 | 1 | 2.02 (1.46–2.78) | <0.001* | ||
| HLP/statin (+) | 500 | 47 (9.4) | 18 (3.6) | 0.41 (0.30–0.55) | <0.001* | 0.50 (0.36–0.68) | <0.001* | 1 | ||
| HLP/statin (-) | 106 | 14 (13.2) | 13 (12.3) | 0.72 (0.43–1.03) | 0.230 | 0.75 (0.44–1.28) | 0.289 | 1.51 (0.83–2.75) | 0.180 | |
| AST (IU/L) | <80 | 1,586 | 186 (11.7) | 103 (6.5) | 1 | -§ | -§ | |||
| ≥80 | 1,193 | 294 (24.6) | 135 (11.3) | 2.03 (1.69–2.44) | <0.001* | -§ | -§ | |||
| ALT (IU/L) | <80 | 1,057 | 119 (11.3) | 74 (7.0) | 1 | -§ | -§ | |||
| ≥80 | 1,722 | 361 (21.0) | 164 (9.5) | 1.67 (1.36–2.05) | <0.001* | -§ | -§ | |||
| FIB-4 | <3.25 | 1,815 | 185 (10.2) | 120 (6.6) | 1 | -§ | -§ | |||
| ≥3.25 | 964 | 295 (30.6) | 118 (12.2) | 3.39 (2.82–4.07) | <0.001* | -§ | -§ | |||
| Liver cirrhosis | No | 2,328 | 339 (14.6) | 170 (7.3) | 1 | 1 | 1 | |||
| Yes | 451 | 141 (31.3) | 68 (15.1) | 2.43 (1.99–2.95) | <0.001* | 2.27 (1.81–2.85) | <0.001* | 2.27 (1.81–2.85) | <0.001* | |
| eGFR (mL/min/1.73m2) | ≥60 | 2,594 | 446 (17.2) | 201 (7.8) | 1 | - | - | |||
| <60 | 185 | 34 (18.4) | 37 (20.0) | 1.03 (0.73–1.45) | 0.871 | - | - | |||
| HCV RNA (IU/mL) | ≤8,000,000 | 2,078 | 370 (17.8) | 182 (8.8) | 1 | 1 | 1 | |||
| >8,000,000 | 332 | 38 (11.5) | 16 (4.8) | 0.66 (0.48–0.93) | 0.016* | 0.73 (0.51–1.04) | 0.079 | 0.73 (0.51–1.04) | 0.079 | |
| HCV genotype | Non-1 | 855 | 126 (14.7) | 82 (9.6) | 1 | 1 | 1 | |||
| 1 | 1,753 | 320 (18.3) | 133 (7.6) | 1.24 (1.01–1.52) | 0.039* | 1.30 (1.04–1.63) | 0.022* | 1.30 (1.04–1.63) | 0.022* | |
§ Due to AST, ALT and FIB-4 were associated with composition in the diagnosis of LC, we did not put these variables in the multivariate analysis.
BMI, body mass index; DM, diabetes mellitus; AST, aspartate aminotransferase; ALT, alanine aminotransferase; eGFR, estimated glomerular filtration rate; FIB-4, fibrosis index based on the 4 factors; eGFR, estimated glomerular filtration rate; HCC, hepatocellular carcinoma; SHR, sub-distribution hazard ratio.
Abbreviations
REFERENCES
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,291 View
- 114 Download
- ORCID iDs
-
Ming-Jong Bair

https://orcid.org/0000-0001-7069-5459Ming-Lung Yu

https://orcid.org/0000-0001-8145-1900 - Related articles
-
Development and prognosis of hepatocellular carcinoma in patients with diabetes2023 January;29(1)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement1
Supplement1 Print
Print



