2. Suh DJ, Jeong SH. Current status of hepatitis C virus infection in Korea. Intervirology 2006;49:70-75.


3. Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol 2014;61(1 Suppl):S45-S57.


4. Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of agespecific antibody to HCV seroprevalence. Hepatology 2013;57:1333-1342.


6. Jeong TH, Jeon TH. PCR prevalence and risk factors of hepatitis C virus infection in the adult population of Ulsan. J Korean Acad Fam Med 1998;19:364-373.
7. Na HY, Park MH, Park KS, Sohn YH, Joo YE, Kim SJ. Geographic characteristics of positivity of anti - HCV and Chonnam province: survey data of 6, 790 health screenees. Korean J Gastroenterol 2001;38:177-184.
8. Park KS, Lee YS, Lee SG, Hwang JY, Chung WJ, Cho KB, et al. A study on markers of viral hepatitis in adults living in Daegu and Gyungbuk area. Korean J Gastroenterol 2003;41:473-479.
9. Seo WT, Lee SS. A study on positive rate of HBs Ag, HBs Ab and anti-HCV in Korean adults. Korean J Blood Transfus 1998;9:259-272.
10. Shin HR. Epidemiology of hepatitis C virus in Korea. Intervirology 2006;49:18-22.


11. Kim do Y, Kim IH, Jeong SH, Cho YK, Lee JH, Jin YJ, et al. A nationwide seroepidemiology of hepatitis C virus infection in South Korea. Liver Int 2013;33:586-594.


13. Oh HB, Hwang YS, Cho YJ, Kim DS, Kim SI. Experience of anti-HCV antibody immunoblot test in Korean blood donors. Korean J Blood Transfus 1997;8:1-8.
16. Moriya T, Sasaki F, Mizui M, Ohno N, Mohri H, Mishiro S, et al. Transmission of hepatitis C virus from mothers to infants: its frequency and risk factors revisited. Biomed Pharmacother 1995;49:59-64.


17. Okamoto M, Nagata I, Murakami J, Kaji S, Iitsuka T, Hoshika T, et al. Prospective reevaluation of risk factors in mother-to-child transmission of hepatitis C virus: high virus load, vaginal delivery, and negative anti-NS4 antibody. J Infect Dis 2000;182:1511-1514.


18. Claret G, Noguera A, Esteva C, Munoz-Almagro C, Sanchez E, Fortuny C. Mother-to-child transmission of hepatitis C virus infection in Barcelona, Spain: a prospective study. Eur J Pediatr 2007;166:1297-1299.


19. Kim YW, Lee JM, Kim GJ, Lee HM, Kim SY, Lee JS, et al. Hepatitis C virus infection in pregnancy. Korean J Obstet Gynecol 2000;43:597-603.
20. Kang MJ, Kim HJ, Park KJ, Kang KH, Ahn HS. Prevalence of HCV infection in pregnant women and vertical transmission. Korean J Obstet Gynecol 2004;47:2045-2050.
21. Lee JM, Lee JM, Yoo HS, Jang UK, Kim DJ, Kim YB, et al. The prevalence of anti-HCV positivity in healthy Korean children. Korean J Hepatol 1996;2:160-165.
22. Kim HS, Choo DH. Prevalence of hepatitis C, B and human immunodeficiency virus among drug users and chronic alcoholic patients in Korea. Korean J Med 1997;52:754-762.
23. Lee SW, Kim SY, Kim JK. Seropositivity of anti-HCV in intravenous drug abusers. J Korean Acad Fam Med 1997;18:1508-1518.
24. Yun H, Kim D, Kim S, Kang S, Jeong S, Cheon Y, et al. High prevalence of HBV and HCV infection among intravenous drug users in Korea. J Med Virol 2008;80:1570-1575.


25. Min JA, Yoon Y, Lee HJ, Choi J, Kwon M, Kim K, et al. Prevalence and associated clinical characteristics of hepatitis B, C, and HIV infections among injecting drug users in Korea. J Med Virol 2013;85:575-582.


26. Macias J, Palacios RB, Claro E, Vargas J, Vergara S, Mira JA, et al. High prevalence of hepatitis C virus infection among noninjecting drug users: association with sharing the inhalation implements of crack. Liver Int 2008;28:781-786.


27. Kim H, Kim KT, Yoo JH, Kim BI, Lee SJ, Lee EJ, et al. Prevalence and risk factors of hepatitis C virus infection in chronic hamodialysis patients(multi-center study). Korean J Med 1997;52:833-840.
28. Shin YH, Kim HK, Choi SD, Kim YS, Shin HS, Won YJ, et al. Prevalence of anti-HCV in hemodialysis patients in Taegu and Kyeongbuk, Korea. Korean J Med 1998;54:640-646.
29. ESRD Registry Committee; Korean Society of Nephrology. Current renal replacement therapy in Korea: Insan memorial dialysis registry, 2014. Kidney Res Clin Pract 2015;117-136.


30. Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C Virus prevalence among patients infected with Human Immunodeficiency Virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis 2002;34:831-837.


31. Kim O, Kim SS, Park MS, Suh SD, Lee MW, Kim KS, et al. Seroprevalence of sexually transmitted viruses in Korean populations including HIV-seropositive individuals. Int J STD AIDS 2003;14:46-49.


33. Kim SY, Kook JH, Choi IS, Kim SJ, Kook H, Hwang TJ. Viral hepatitis and change of lymphocyte subpopulation in hemophiliacs in Chonnam KwangJu area. Korean J Blood Transfus 2002;13:43-51.
34. Korea Hemophilia Association. 2012 Korean hemophilia annual report. Seoul: Korea Hemophilia Association; 2012.
35. Choi SH. The prevalence of hepatitis C virus infection in leprous patients. Korean J Gastroenterol 1997;30:486-494.
36. Lavanchy D. The global burden of hepatitis C. Liver Int 2009;29 Suppl 1:74-81.

37. Williams IT, Bell BP, Kuhnert W, Alter MJ. Incidence and transmission patterns of acute hepatitis C in the United States, 1982-2006. Arch Intern Med 2011;171:242-248.


39. Oh HB, Hwang YS, Kim DS, Kim SI, Lee SY, Han KS. Study on the seroincidence of hepatitic C virus infection among blood donors in Korea. Korean J Blood Transfus 1997;8:33-41.
40. Esteban JI, Sauleda S, Quer J. The changing epidemiology of hepatitis C virus infection in Europe. J Hepatol 2008;48:148-162.


41. Rustgi VK. The epidemiology of hepatitis C infection in the United States. J Gastroenterol 2007;42:513-521.


44. Raimondi S, Bruno S, Mondelli MU, Maisonneuve P. Hepatitis C virus genotype 1b as a risk factor for hepatocellular carcinoma development: a meta-analysis. J Hepatol 2009;50:1142-1154.


46. Busch MP, Glynn SA, Stramer SL, Strong DM, Caglioti S, Wright DJ, et al. A new strategy for estimating risks of transfusion-transmitted viral infections based on rates of detection of recently infected donors. Transfusion 2005;45:254-264.


47. Vermeulen M, Lelie N, Sykes W, Crookes R, Swanevelder J, Gaggia L, et al. Impact of individual-donation nucleic acid testing on risk of human immunodeficiency virus, hepatitis B virus, and hepatitis C virus transmission by blood transfusion in South Africa. Transfusion 2009;49:1115-1125.


48. Shan H, Ren FR, Zhao HY, Zhang YZ, Wen GX, Yao FZ, et al. A multi-Chinese blood center study testing serologic-negative donor samples for hepatitis C virus and human immunodeficiency virus with nucleic acid testing. Transfusion 2007;47:2011-2016.


49. Papatheodoridis G, Hatzakis A. Public health issues of hepatitis C virus infection. Best Pract Res Clin Gastroenterol 2012;26:371-380.


51. Kermode M. Unsafe injections in low-income country health settings: need for injection safety promotion to prevent the spread of blood-borne viruses. Health Promot Int 2004;19:95-103.


52. Deterding K, Wiegand J, Gruner N, Hahn A, Jackel E, Jung MC, et al. The German Hep-Net acute hepatitis C cohort: impact of viral and host factors on the initial presentation of acute hepatitis C virus infection. Z Gastroenterol 2009;47:531-540.


53. Gutelius B, Perz JF, Parker MM, Hallack R, Stricof R, Clement EJ, et al. Multiple clusters of hepatitis virus infections associated with anesthesia for outpatient endoscopy procedures. Gastroenterology 2010;139:163-170.


54. Hayes MO, Harkness GA. Body piercing as a risk factor for viral hepatitis: an integrative research review. Am J Infect Control 2001;29:271-274.


55. Ernst E, Sherman KJ. Is acupuncture a risk factor for hepatitis? Systematic review of epidemiological studies. J Gastroenterol Hepatol 2003;18:1231-1236.


56. Jafari S, Copes R, Baharlou S, Etminan M, Buxton J. Tattooing and the risk of transmission of hepatitis C: a systematic review and meta-analysis. Int J Infect Dis 2010;14:e928-e940.


57. Alter MJ. Epidemiology of hepatitis C. Hepatology 1997;26(3 Suppl 1):62S-65S.

58. Tomkins SE, Elford J, Nichols T, Aston J, Cliffe SJ, Roy K, et al. Occupational transmission of hepatitis C in healthcare workers and factors associated with seroconversion: UK surveillance data. J Viral Hepat 2012;19:199-204.


59. Puro V, Petrosillo N, Ippolito G. Risk of hepatitis C seroconversion after occupational exposures in health care workers. Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections. Am J Infect Control 1995;23:273-277.


60. Lanphear BP, Linnemann CC Jr, Cannon CG, DeRonde MM, Pendy L, Kerley LM. Hepatitis C virus infection in healthcare workers: risk of exposure and infection. Infect Control Hosp Epidemiol 1994;15:745-750.


61. Ryoo SM, Kim WY, Kim W, Lim KS, Lee CC, Woo JH. Transmission of hepatitis C virus by occupational percutaneous injuries in South Korea. J Formos Med Assoc 2012;111:113-117.


62. Tohme RA, Holmberg SD. Is sexual contact a major mode of hepatitis C virus transmission? Hepatology 2010;52:1497-1505.


63. Yaphe S, Bozinoff N, Kyle R, Shivkumar S, Pai NP, Klein M. Incidence of acute hepatitis C virus infection among men who have sex with men with and without HIV infection: a systematic review. Sex Transm Infect 2012;88:558-564.


65. Roberts EA, Yeung L. Maternal-infant transmission of hepatitis C virus infection. Hepatology 2002;36(5 Suppl 1):S106-S113.


66. Indolfi G, Resti M. Perinatal transmission of hepatitis C virus infection. J Med Virol 2009;81:836-843.


67. European Paediatric Hepatitis C Virus Network. A significant sex--but not elective cesarean section--effect on mother-to-child transmission of hepatitis C virus infection. J Infect Dis 2005;192:1872-1879.


68. Ghamar Chehreh ME, Tabatabaei SV, Khazanehdari S, Alavian SM. Effect of cesarean section on the risk of perinatal transmission of hepatitis C virus from HCV-RNA’╝ŗ/HIV- mothers: a meta-analysis. Arch Gynecol Obstet 2011;283:255-260.


69. European Paediatric Hepatitis C Virus Network. Effects of mode of delivery and infant feeding on the risk of mother-to-child transmission of hepatitis C virus. European Paediatric Hepatitis C Virus Network. BJOG 2001;108:371-377.

70. Indolfi G, Nesi A, Resti M. Intrafamilial transmission of hepatitis C virus. J Med Virol 2013;85:608-614.


71. Seong MH, Kil H, Kim YS, Bae SH, Lee YJ, Lee HC, et al. Clinical and epidemiological features of hepatitis C virus infection in South Korea: a prospective, multicenter cohort study. J Med Virol 2013;85:1724-1733.


72. Farci P, Alter HJ, Wong D, Miller RH, Shih JW, Jett B, et al. A long-term study of hepatitis C virus replication in non-A, non-B hepatitis. N Engl J Med 1991;325:98-104.


73. Maasoumy B, Wedemeyer H. Natural history of acute and chronic hepatitis C. Best Pract Res Clin Gastroenterol 2012;26:401-412.


75. Santantonio T, Wiegand J, Gerlach JT. Acute hepatitis C: current status and remaining challenges. J Hepatol 2008;49:625-633.


76. Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis 2000;20:17-35.


77. Gerlach JT, Diepolder HM, Zachoval R, Gruener NH, Jung MC, Ulsenheimer A, et al. Acute hepatitis C: high rate of both spontaneous and treatment-induced viral clearance. Gastroenterology 2003;125:80-88.


78. Tremolada F, Casarin C, Alberti A, Drago C, Tagger A, Ribero ML, et al. Long-term follow-up of non-A, non-B (type C) post-transfusion hepatitis. J Hepatol 1992;16:273-281.


79. Lehmann M, Meyer MF, Monazahian M, Tillmann HL, Manns MP, Wedemeyer H. High rate of spontaneous clearance of acute hepatitis C virus genotype 3 infection. J Med Virol 2004;73:387-391.


80. Hofer H, Watkins-Riedel T, Janata O, Penner E, Holzmann H, SteindlMunda P, et al. Spontaneous viral clearance in patients with acute hepatitis C can be predicted by repeated measurements of serum viral load. Hepatology 2003;37:60-64.


81. Kim KA, Lee JS, Yang JH, Moon YS, Lee WJ. Natural history of acute symptomatic hepatitis C in Korea. Korean J Gastroenterol 2005;46:105-109.

82. Kim JY, Won JE, Jeong SH, Park SJ, Hwang SG, Kang SK, et al. Acute hepatitis C in Korea: different modes of infection, high rate of spontaneous recovery, and low rate of seroconversion. J Med Virol 2011;83:1195-1202.


84. Tillmann HL, Thompson AJ, Patel K, Wiese M, Tenckhoff H, Nischalke HD, et al. A polymorphism near IL28B is associated with spontaneous clearance of acute hepatitis C virus and jaundice. Gastroenterology 2010;139:1586-1592 1592.e1.


85. Rao HY, Sun DG, Jiang D, Yang RF, Guo F, Wang JH, et al. IL28B genetic variants and gender are associated with spontaneous clearance of hepatitis C virus infection. J Viral Hepat 2012;19:173-181.


86. Tong MJ, el-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med 1995;332:1463-1466.


87. Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology 2008;48:418-431.


88. Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet 1997;349:825-832.


90. Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, et al. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology 2006;43:1303-1310.


91. Gomez EV, Rodriguez YS, Bertot LC, Gonzalez AT, Perez YM, Soler EA, et al. The natural history of compensated HCV-related cirrhosis: a prospective long-term study. J Hepatol 2013;58:434-444.


92. Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology 1997;112:463-472.


93. Sinn DH, Paik SW, Kil JS, Kang P, Song SM, Gwak GY, et al. Incidence and risk factors for disease progression in Korean patients with chronic hepatitis C. Clin Mol Hepatol 2007;13(Suppl):S19.
94. Lee SS, Jeong SH, Jang ES, Kim YS, Lee YJ, Jung EU, et al. Prospective cohort study on the outcomes of hepatitis C virus-related cirrhosis in South Korea. J Gastroenterol Hepatol 2015;30:1281-1287.


95. Zarski JP, Mc Hutchison J, Bronowicki JP, Sturm N, Garcia-Kennedy R, Hodaj E, et al. Rate of natural disease progression in patients with chronic hepatitis C. J Hepatol 2003;38:307-314.


96. Harris DR, Gonin R, Alter HJ, Wright EC, Buskell ZJ, Hollinger FB, et al. The relationship of acute transfusion-associated hepatitis to the development of cirrhosis in the presence of alcohol abuse. Ann Intern Med 2001;134:120-124.


97. Wiley TE, McCarthy M, Breidi L, McCarthy M, Layden TJ. Impact of alcohol on the histological and clinical progression of hepatitis C infection. Hepatology 1998;28:805-809.


98. Noda K, Yoshihara H, Suzuki K, Yamada Y, Kasahara A, Hayashi N, et al. Progression of type C chronic hepatitis to liver cirrhosis and hepatocellular carcinoma--its relationship to alcohol drinking and the age of transfusion. Alcohol Clin Exp Res 1996;20(1 Suppl):95A-100A.

99. Ortiz V, Berenguer M, Rayon JM, Carrasco D, Berenguer J. Contribution of obesity to hepatitis C-related fibrosis progression. Am J Gastroenterol 2002;97:2408-2414.


100. Ohki T, Tateishi R, Sato T, Masuzaki R, Imamura J, Goto T, et al. Obesity is an independent risk factor for hepatocellular carcinoma development in chronic hepatitis C patients. Clin Gastroenterol Hepatol 2008;6:459-464.


101. Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology 2001;33:1358-1364.


102. Ohata K, Hamasaki K, Toriyama K, Matsumoto K, Saeki A, Yanagi K, et al. Hepatic steatosis is a risk factor for hepatocellular carcinoma in patients with chronic hepatitis C virus infection. Cancer 2003;97:3036-3043.


103. Poynard T, Ratziu V, Charlotte F, Goodman Z, McHutchison J, Albrecht J. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis c. J Hepatol 2001;34:730-739.


104. Imazeki F, Yokosuka O, Fukai K, Hiraide A, Saisho H. Significance of prior hepatitis B virus infection in the development of hepatocellular carcinoma in patients with chronic hepatitis C. Dig Dis Sci 2003;48:1786-1792.


105. Tsai JF, Jeng JE, Ho MS, Chang WY, Lin ZY, Tsai JH. Independent and additive effect modification of hepatitis C and B viruses infection on the development of chronic hepatitis. J Hepatol 1996;24:271-276.


106. Vento S, Garofano T, Renzini C, Cainelli F, Casali F, Ghironzi G, et al. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med 1998;338:286-290.


107. Ghany MG, Strader DB, Thomas DL, Seeff LB. American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 2009;49:1335-1374.


109. Moyer VA. U.S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2013;159:349-357.


111. Nakamura J, Terajima K, Aoyagi Y, Akazawa K. Cost-effectiveness of the national screening program for hepatitis C virus in the general population and the high-risk groups. Tohoku J Exp Med 2008;215:33-42.


112. Sroczynski G, Esteban E, Conrads-Frank A, Schwarzer R, Muhlberger N, Wright D, et al. Long-term effectiveness and cost-effectiveness of screening for hepatitis C virus infection. Eur J Public Health 2009;19:245-253.


113. Ki M, Choi HY, Jang ES, Jeong SH. Direct medical cost for hepatitis C virus infection in South Korea, 2009-2013 [Abstract]. The Liver Week 2015;2015:110A.
114. Kim DY, Han KH, Jun B, Kim TH, Park S, Ward T, et al. Cost-effectiveness of one-time screening for HCV in South Korea [Abstract]. The Liver Week 2015;2015:23A.
115. Pawlotsky JM. Molecular diagnosis of viral hepatitis. Gastroenterology 2002;122:1554-1568.


116. Colin C, Lanoir D, Touzet S, Meyaud-Kraemer L, Bailly F, Trepo C, et al. Sensitivity and specificity of third-generation hepatitis C virus antibody detection assays: an analysis of the literature. J Viral Hepat 2001;8:87-95.


118. Pawlotsky JM. Use and interpretation of virological tests for hepatitis C. Hepatology 2002;36(5 Suppl 1):S65-S73.


119. Pawlotsky JM, Lonjon I, Hezode C, Raynard B, Darthuy F, Remire J, et al. What strategy should be used for diagnosis of hepatitis C virus infection in clinical laboratories? Hepatology 1998;27:1700-1702.


120. Stramer SL, Caglioti S, Strong DM. NAT of the United States and Canadian blood supply. Transfusion 2000;40:1165-1168.


121. Alter MJ, Kuhnert WL, Finelli L. Centers for Disease Control and Prevention. Guidelines for laboratory testing and result reporting of antibody to hepatitis C virus. Centers for Disease Control and Prevention. MMWR Recomm Rep 2003;52(RR-3):1-13 15;quiz CE11-14.
123. Lee SR, Kardos KW, Schiff E, Berne CA, Mounzer K, Banks AT, et al. Evaluation of a new, rapid test for detecting HCV infection, suitable for use with blood or oral fluid. J Virol Methods 2011;172:27-31.


124. European Association for Study of Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol 2014;60:392-420.


125. Alter MJ, Margolis HS, Krawczynski K, Judson FN, Mares A, Alexander WJ, et al. The natural history of community-acquired hepatitis C in the United States. The Sentinel Counties Chronic non-A, non-B Hepatitis Study Team. N Engl J Med 1992;327:1899-1905.


126. Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 2001;345:41-52.


127. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol 2011;55:245-264.


129. Meyer zum Buschenfelde KH, Gerken G, Manns M. Hepatitis C virus (HCV) and autoimmune liver diseases. Arch Virol Suppl 1992;4:201-204.


130. Scott JD, Gretch DR. Molecular diagnostics of hepatitis C virus infection: a systematic review. JAMA 2007;297:724-732.


131. Sarrazin C, Teuber G, Kokka R, Rabenau H, Zeuzem S. Detection of residual hepatitis C virus RNA by transcription-mediated amplification in patients with complete virologic response according to polymerase chain reaction-based assays. Hepatology 2000;32(4 Pt 1):818-823.


132. Martinot-Peignoux M, Boyer N, Le Breton V, Le Guludec G, Castelnau C, Akremi R, et al. A new step toward standardization of serum hepatitis C virus-RNA quantification in patients with chronic hepatitis C. Hepatology 2000;31:726-729.


133. Pradat P, Chossegros P, Bailly F, Pontisso P, Saracco G, Sauleda S, et al. Comparison between three quantitative assays in patients with chronic hepatitis C and their relevance in the prediction of response to therapy. J Viral Hepat 2000;7:203-210.


136. Vermehren J, Yu ML, Monto A, Yao JD, Anderson C, Bertuzis R, et al. Multi-center evaluation of the Abbott RealTime HCV assay for monitoring patients undergoing antiviral therapy for chronic hepatitis C. J Clin Virol 2011;52:133-137.


137. Pawlotsky JM, Bouvier-Alias M, Hezode C, Darthuy F, Remire J, Dhumeaux D. Standardization of hepatitis C virus RNA quantification. Hepatology 2000;32:654-659.


138. Saldanha J, Lelie N, Heath A. Establishment of the first international standard for nucleic acid amplification technology (NAT) assays for HCV RNA. WHO Collaborative Study Group. Vox Sang 1999;76:149-158.


140. Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol 2005;5:215-229.


142. Nguyen TT, Sedghi-Vaziri A, Wilkes LB, Mondala T, Pockros PJ, Lindsay KL, et al. Fluctuations in viral load (HCV RNA) are relatively insignificant in untreated patients with chronic HCV infection. J Viral Hepat 1996;3:75-78.


143. Ferreira-Gonzalez A, Shiffman ML. Use of diagnostic testing for managing hepatitis C virus infection. Semin Liver Dis 2004;24 Suppl 2:9-18.


145. Simmonds P, Bukh J, Combet C, Deleage G, Enomoto N, Feinstone S, et al. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology 2005;42:962-973.


146. Chevaliez S, Pawlotsky JM. Diagnosis and management of chronic viral hepatitis: antigens, antibodies and viral genomes. Best Pract Res Clin Gastroenterol 2008;22:1031-1048.


147. Smith DB, Mellor J, Jarvis LM, Davidson F, Kolberg J, Urdea M, et al. Variation of the hepatitis C virus 5ŌĆÖ non-coding region: implications for secondary structure, virus detection and typing. The International HCV Collaborative Study Group. J Gen Virol 1995;76(Pt 7):1749-1761.


148. Park JC, Kim JM, Kwon OJ, Lee KR, Chai YG, Oh HB. Development and clinical evaluation of a microarray for hepatitis C virus genotyping. J Virol Methods 2010;163:269-275.


152. Cai Q, Zhao Z, Liu Y, Shao X, Gao Z. Comparison of three different HCV genotyping methods: core, NS5B sequence analysis and line probe assay. Int J Mol Med 2013;31:347-352.


153. McCormick AL, Macartney MJ, Abdi-Abshir I, Labbett W, Smith C, Irish D, et al. Evaluation of sequencing of HCV core/E1, NS5A and NS5B as a genotype predictive tool in comparison with commercial assays targeting 5ŌĆÖUTR. J Clin Virol 2015;66:56-59.


157. Lenz O, Fevery B, Verbinnen T, Tambuyzer L, Vijgen L, Peeters M, et al. Resistance analyses of HCV isolates from patients treated with simeprevir in phase 2b/3 studies. [Abstract]. Hepatology 2013;58:743A.

158. Lenz O, Verbinnen T, Fevery B, Tambuyzer L, Vijgen L, Peeters M, et al. Virology analyses of HCV isolates from genotype 1-infected patients treated with simeprevir plus PegIFN-a/ribavirin in Phase IIb/III studies. J Hepatol 2015;62:1008-1014.


159. Suzuki F, Sezaki H, Akuta N, Suzuki Y, Seko Y, Kawamura Y, et al. Prevalence of hepatitis C virus variants resistant to NS3 protease inhibitors or the NS5A inhibitor (BMS-790052) in hepatitis patients with genotype 1b. J Clin Virol 2012;54:352-354.


160. Karino Y, Toyota J, Ikeda K, Suzuki F, Chayama K, Kawakami Y, et al. Characterization of virologic escape in hepatitis C virus genotype 1b patients treated with the direct-acting antivirals daclatasvir and asunaprevir. J Hepatol 2013;58:646-654.


161. Pawlotsky JM. Drug resistance: prevalence and clinical implications during the treatment of chronic hepatitis C infection. Clin Liver Disease 2012;1:58-61.

162. Halfon P, Sarrazin C. Future treatment of chronic hepatitis C with direct acting antivirals: is resistance important? Liver Int 2012;32 Suppl 1:79-87.

164. McPhee F, Hernandez D, Yu F, Ueland J, Monikowski A, Carifa A, et al. Resistance analysis of hepatitis C virus genotype 1 prior treatment null responders receiving daclatasvir and asunaprevir. Hepatology 2013;58:902-911.


165. Lok AS, Gardiner DF, Lawitz E, Martorell C, Everson GT, Ghalib R, et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med 2012;366:216-224.


166. Chayama K, Takahashi S, Toyota J, Karino Y, Ikeda K, Ishikawa H, et al. Dual therapy with the nonstructural protein 5A inhibitor, daclatasvir, and the nonstructural protein 3 protease inhibitor, asunaprevir, in hepatitis C virus genotype 1b-infected null responders. Hepatology 2012;55:742-748.


168. Nguyen LT, Gray E, Dean J, Carr M, Connell J, De Gascun C, et al. Baseline prevalence and emergence of protease inhibitor resistance mutations following treatment in chronic HCV genotype 1-infected individuals. Antivir Ther 2015;[Epub ahead of print].

171. Sarrazin C. The importance of resistance to direct antiviral drugs in HCV infection in clinical practice. J Hepatol 2015;[Epub ahead of print].

172. Lawitz EJ, Gruener D, Hill JM, Marbury T, Moorehead L, Mathias A, et al. A phase 1, randomized, placebo-controlled, 3-day, dose-ranging study of GS-5885, an NS5A inhibitor, in patients with genotype 1 hepatitis C. J Hepatol 2012;57:24-31.


174. Alter MJ. The epidemiology of acute and chronic hepatitis C. Clin Liver Dis 1997;1:559-568 vi-vii.


175. Public Health Service. Updated U.S. Public Health Service Guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep 2001;50(RR-11):1-52.

176. Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Teo CG, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep 2012;61(RR-4):1-32.
177. Mitsui T, Iwano K, Masuko K, Yamazaki C, Okamoto H, Tsuda F, et al. Hepatitis C virus infection in medical personnel after needlestick accident. Hepatology 1992;16:1109-1114.


178. Kubitschke A, Bahr MJ, Aslan N, Bader C, Tillmann HL, Sarrazin C, et al. Induction of hepatitis C virus (HCV)-specific T cells by needle stick injury in the absence of HCV-viraemia. Eur J Clin Invest 2007;37:54-64.


179. Kleiner DE. The liver biopsy in chronic hepatitis C: a view from the other side of the microscope. Semin Liver Dis 2005;25:52-64.


180. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 1996;24:289-293.


181. Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995;22:696-699.


182. Park YN, Kim Hg, Chon CY, Park JB, Sohn JH, Yang SH, et al. Histological grading and staging of chronic hepatitis standardized guideline proposed by the Korean study group for the pathology of digestive diseases. Korean J Pathol 1999;33:337-346.
183. Levine RA, Sanderson SO, Ploutz-Snyder R, Murray F, Kay E, Hegarty J, et al. Assessment of fibrosis progression in untreated irish women with chronic hepatitis C contracted from immunoglobulin anti-D. Clin Gastroenterol Hepatol 2006;4:1271-1277.


184. Thomas DL, Seeff LB. Natural history of hepatitis C. Clin Liver Dis 2005;9:383-398 vi.


185. Wong JB, Koff RS. Watchful waiting with periodic liver biopsy versus immediate empirical therapy for histologically mild chronic hepatitis C. A cost-effectiveness analysis. Ann Intern Med 2000;133:665-675.


186. Martinot-Peignoux M, Boyer N, Cazals-Hatem D, Pham BN, Gervais A, Le Breton V, et al. Prospective study on anti-hepatitis C virus-positive patients with persistently normal serum alanine transaminase with or without detectable serum hepatitis C virus RNA. Hepatology 2001;34:1000-1005.


187. Shiffman ML, Diago M, Tran A, Pockros P, Reindollar R, Prati D, et al. Chronic hepatitis C in patients with persistently normal alanine transaminase levels. Clin Gastroenterol Hepatol 2006;4:645-652.


188. Boccato S, Pistis R, Noventa F, Guido M, Benvegnu L, Alberti A. Fibrosis progression in initially mild chronic hepatitis C. J Viral Hepat 2006;13:297-302.


189. Yano M, Kumada H, Kage M, Ikeda K, Shimamatsu K, Inoue O, et al. The long-term pathological evolution of chronic hepatitis C. Hepatology 1996;23:1334-1340.


190. Fontaine H, Nalpas B, Poulet B, Carnot F, Zylberberg H, Brechot C, et al. Hepatitis activity index is a key factor in determining the natural history of chronic hepatitis C. Hum Pathol 2001;32:904-909.


192. Poynard T, Ratziu V, McHutchison J, Manns M, Goodman Z, Zeuzem S, et al. Effect of treatment with PegIFN-a or interferon alfa- 2b and ribavirin on steatosis in patients infected with hepatitis C. Hepatology 2003;38:75-85.


193. Olynyk JK, Reddy KR, Di Bisceglie AM, Jeffers LJ, Parker TI, Radick JL, et al. Hepatic iron concentration as a predictor of response to interferon alfa therapy in chronic hepatitis C. Gastroenterology 1995;108:1104-1109.


194. Westin J, Lagging M, Dhillon AP, Norkrans G, Romero AI, Pawlotsky JM, et al. Impact of hepatic steatosis on viral kinetics and treatment outcome during antiviral treatment of chronic HCV infection. J Viral Hepat 2007;14:29-35.

195. Patton HM, Patel K, Behling C, Bylund D, Blatt LM, Vallee M, et al. The impact of steatosis on disease progression and early and sustained treatment response in chronic hepatitis C patients. J Hepatol 2004;40:484-490.


196. Fontana RJ, Israel J, LeClair P, Banner BF, Tortorelli K, Grace N, et al. Iron reduction before and during interferon therapy of chronic hepatitis C: results of a multicenter, randomized, controlled trial. Hepatology 2000;31:730-736.


197. Reiss G, Keeffe EB. Role of liver biopsy in the management of chronic liver disease: selective rather than routine. Rev Gastroenterol Disord 2005;5:195-205.

198. Crockett SD, Kaltenbach T, Keeffe EB. Do we still need a liver biopsy? Are the serum fibrosis tests ready for prime time? Clin Liver Dis 2006;10:513-534 viii.


199. Dienstag JL. The role of liver biopsy in chronic hepatitis C. Hepatology 2002;36:S152-160.


200. Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology 2000;32:477-481.


201. Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 2002;97:2614-2618.


202. Rockey DC, Bissell DM. Noninvasive measures of liver fibrosis. Hepatology 2006;43(2 Suppl 1):S113-S120.


203. Poynard T, Ngo Y, Munteanu M, Thabut D, Massard J, Moussalli J, et al. Biomarkers of liver injury for hepatitis clinical trials: a meta-analysis of longitudinal studies. Antivir Ther 2010;15:617-631.


204. Colletta C, Smirne C, Fabris C, Toniutto P, Rapetti R, Minisini R, et al. Value of two noninvasive methods to detect progression of fibrosis among HCV carriers with normal aminotransferases. Hepatology 2005;42:838-845.


205. Halfon P, Bourliere M, Deydier R, Botta-Fridlund D, Renou C, Tran A, et al. Independent prospective multicenter validation of biochemical markers (fibrotest-actitest) for the prediction of liver fibrosis and activity in patients with chronic hepatitis C: the fibropaca study. Am J Gastroenterol 2006;101:547-555.


206. Parkes J, Guha IN, Roderick P, Harris S, Cross R, Manos MM, et al. Enhanced Liver Fibrosis (ELF) test accurately identifies liver fibrosis in patients with chronic hepatitis C. J Viral Hepat 2011;18:23-31.


207. Sheth SG, Flamm SL, Gordon FD, Chopra S. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol 1998;93:44-48.


208. Forns X, Ampurdanes S, Llovet JM, Aponte J, Quinto L, Martinez-Bauer E, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology 2002;36:986-992.


209. Shaheen AA, Myers RP. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis C-related fibrosis: a systematic review. Hepatology 2007;46:912-921.


211. Adams LA, Bulsara M, Rossi E, DeBoer B, Speers D, George J, et al. Hepascore: an accurate validated predictor of liver fibrosis in chronic hepatitis C infection. Clin Chem 2005;51:1867-1873.


212. Boursier J, Bacq Y, Halfon P, Leroy V, de Ledinghen V, de Muret A, et al. Improved diagnostic accuracy of blood tests for severe fibrosis and cirrhosis in chronic hepatitis C. Eur J Gastroenterol Hepatol 2009;21:28-38.


213. Patel K, Benhamou Y, Yoshida EM, Kaita KD, Zeuzem S, Torbenson M, et al. An independent and prospective comparison of two commercial fibrosis marker panels (HCV FibroSURE and FIBROSpect II) during albinterferon alfa-2b combination therapy for chronic hepatitis C. J Viral Hepat 2009;16:178-186.


214. Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518-526.


215. Park GJ, Lin BP, Ngu MC, Jones DB, Katelaris PH. Aspartate aminotransferase: alanine aminotransferase ratio in chronic hepatitis C infection: is it a useful predictor of cirrhosis? J Gastroenterol Hepatol 2000;15:386-390.


216. Giannini E, Risso D, Botta F, Chiarbonello B, Fasoli A, Malfatti F, et al. Validity and clinical utility of the aspartate aminotransferasealanine aminotransferase ratio in assessing disease severity and prognosis in patients with hepatitis C virus-related chronic liver disease. Arch Intern Med 2003;163:218-224.


217. Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated metaanalysis. Hepatology 2011;53:726-736.


218. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317-1325.


219. Castera L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 2005;128:343-350.


220. Castera L. Transient elastography and other noninvasive tests to assess hepatic fibrosis in patients with viral hepatitis. J Viral Hepat 2009;16:300-314.


223. Arena U, Vizzutti F, Corti G, Ambu S, Stasi C, Bresci S, et al. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology 2008;47:380-384.


224. Sagir A, Erhardt A, Schmitt M, Haussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology 2008;47:592-595.


225. Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol 2008;48:835-847.


226. Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 2005;41:48-54.


227. Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology 2008;134:960-974.


228. Degos F, Perez P, Roche B, Mahmoudi A, Asselineau J, Voitot H, et al. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J Hepatol 2010;53:1013-1021.


229. Talwalkar JA, Yin M, Fidler JL, Sanderson SO, Kamath PS, Ehman RL. Magnetic resonance imaging of hepatic fibrosis: emerging clinical applications. Hepatology 2008;47:332-342.


230. Friedrich-Rust M, Nierhoff J, Lupsor M, Sporea I, Fierbinteanu-Braticevici C, Strobel D, et al. Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J Viral Hepat 2012;19:e212-e219.


231. Lee Y, Lee JM, Lee JE, Lee KB, Lee ES, Yoon JH, et al. MR elastography for noninvasive assessment of hepatic fibrosis: reproducibility of the examination and reproducibility and repeatability of the liver stiffness value measurement. J Magn Reson Imaging 2014;39:326-331.


232. Swain MG, Lai MY, Shiffman ML, Cooksley WG, Zeuzem S, Dieterich DT, et al. A sustained virologic response is durable in patients with chronic hepatitis C treated with PegIFN-a alfa-2a and ribavirin. Gastroenterology 2010;139:1593-1601.


233. Manns M, Pol S, Jacobson IM, Marcellin P, Gordon SC, Peng CY, et al. All-oral daclatasvir plus asunaprevir for hepatitis C virus genotype 1b: a multinational, phase 3, multicohort study. Lancet 2014;384:1597-1605.


234. Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med 2014;370:211-221.


235. Shiratori Y, Imazeki F, Moriyama M, Yano M, Arakawa Y, Yokosuka O, et al. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med 2000;132:517-524.


236. Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, et al. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology 2002;122:1303-1313.


237. Pradat P, Tillmann HL, Sauleda S, Braconier JH, Saracco G, Thursz M, et al. Long-term follow-up of the hepatitis C HENCORE cohort: response to therapy and occurrence of liver-related complications. J Viral Hepat 2007;14:556-563.


238. Shiratori Y, Ito Y, Yokosuka O, Imazeki F, Nakata R, Tanaka N, et al. Antiviral therapy for cirrhotic hepatitis C: association with reduced hepatocellular carcinoma development and improved survival. Ann Intern Med 2005;142:105-114.


239. Ogawa E, Furusyo N, Kajiwara E, Takahashi K, Nomura H, Maruyama T, et al. Efficacy of pegylated interferon alpha-2b and ribavirin treatment on the risk of hepatocellular carcinoma in patients with chronic hepatitis C: a prospective, multicenter study. J Hepatol 2013;58:495-501.


240. Deuffic-Burban S, Deltenre P, Louvet A, Canva V, Dharancy S, Hollebecque A, et al. Impact of viral eradication on mortality related to hepatitis C: a modeling approach in France. J Hepatol 2008;49:175-183.


241. Bruno S, Stroffolini T, Colombo M, Bollani S, Benvegnu L, Mazzella G, et al. Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology 2007;45:579-587.


242. Fabrizi F, Dixit V, Messa P. Antiviral therapy of symptomatic HCV-associated mixed cryoglobulinemia: meta-analysis of clinical studies. J Med Virol 2013;85:1019-1027.


243. Feng B, Eknoyan G, Guo ZS, Jadoul M, Rao HY, Zhang W, et al. Effect of interferon-alpha- based antiviral therapy on hepatitis C virus-associated glomerulonephritis: a meta-analysis. Nephrol Dial Transplant 2012;27:640-646.


244. Everson GT, Trotter J, Forman L, Kugelmas M, Halprin A, Fey B, et al. Treatment of advanced hepatitis C with a low accelerating dosage regimen of antiviral therapy. Hepatology 2005;42:255-262.


245. Berenguer M, Palau A, Aguilera V, Ray├│n JM, Juan FS, Prieto M. Clinical benefits of antiviral therapy in patients with recurrent hepatitis C following liver transplantation. Am J Transplant 2008;8:679-687.


246. Hagihara H, Nouso K, Kobayashi Y, Iwasaki Y, Nakamura S, Kuwaki K, et al. Effect of pegylated interferon therapy on intrahepatic recurrence after curative treatment of hepatitis C virus-related hepatocellular carcinoma. Int J Clin Oncol 2011;16:210-220.


247. Singal AK, Freeman DH Jr, Anand BS. Meta-analysis: interferon improves outcomes following ablation or resection of hepatocellular carcinoma. Aliment Pharmacol Ther 2010;32:851-858.


248. Chen J, Florian J, Carter W, Fleischer RD, Hammerstrom TS, Jadhav PR, et al. Earlier sustained virologic response end points for regulatory approval and dose selection of hepatitis C therapies. Gastroenterology 2013;144:1450-1455 e2.


249. Mangia A, Minerva N, Bacca D, Cozzolongo R, Ricci GL, Carretta V, et al. Individualized treatment duration for hepatitis C genotype 1 patients: A randomized controlled trial. Hepatology 2008;47:43-50.


250. Jensen DM, Morgan TR, Marcellin P, Pockros PJ, Reddy KR, Hadziyannis SJ, et al. Early identification of HCV genotype 1 patients responding to 24 weeks PegIFN-a-2a (40 kd)/ribavirin therapy. Hepatology 2006;43:954-960.


251. Yu JW, Wang GQ, Sun LJ, Li XG, Li SC. Predictive value of rapid virological response and early virological response on sustained virological response in HCV patients treated with pegylated interferon alpha-2a and ribavirin. J Gastroenterol Hepatol 2007;22:832-836.


252. Yu ML, Dai CY, Huang JF, Chiu CF, Yang YH, Hou NJ, et al. Rapid virological response and treatment duration for chronic hepatitis C genotype 1 patients: a randomized trial. Hepatology 2008;47:1884-1893.


253. Andriulli A, Mangia A, Iacobellis A, Ippolito A, Leandro G, Zeuzem S. Meta-analysis: the outcome of anti-viral therapy in HCV genotype 2 and genotype 3 infected patients with chronic hepatitis. Aliment Pharmacol Ther 2008;28:397-404.


254. Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002;347:975-982.


255. Davis GL, Wong JB, McHutchison JG, Manns MP, Harvey J, Albrecht J. Early virologic response to treatment with PegIFN-a alfa- 2b plus ribavirin in patients with chronic hepatitis C. Hepatology 2003;38:645-652.


256. Ferenci P, Fried MW, Shiffman ML, Smith CI, Marinos G, Goncales FL Jr, et al. Predicting sustained virological responses in chronic hepatitis C patients treated with PegIFN-a alfa-2a (40 KD)/ribavirin. J Hepatol 2005;43:425-433.


257. Berg T, von Wagner M, Nasser S, Sarrazin C, Heintges T, Gerlach T, et al. Extended treatment duration for hepatitis C virus type 1: comparing 48 versus 72 weeks of PegIFN-a-alfa-2a plus ribavirin. Gastroenterology 2006;130:1086-1097.


258. Pearlman BL, Ehleben C, Saifee S. Treatment extension to 72 weeks of PegIFN-a and ribavirin in hepatitis c genotype 1-infected slow responders. Hepatology 2007;46:1688-1694.


259. Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013;368:1878-1887.


260. Forns X, Lawitz E, Zeuzem S, Gane E, Bronowicki JP, Andreone P, et al. Simeprevir with PegIFN-a and ribavirin leads to high rates of SVR in patients with HCV genotype 1 who relapsed after previous therapy: a phase 3 trial. Gastroenterology 2014;146:1669-1679 e3.


261. Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001;358:958-965.


262. Hadziyannis SJ, Sette H Jr, Morgan TR, Balan V, Diago M, Marcellin P, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med 2004;140:346-355.


263. Thompson AJ, Muir AJ, Sulkowski MS, Ge D, Fellay J, Shianna KV, et al. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology 2010;139:120-129 e18.


264. Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 2009;461:399-401.


266. von Wagner M, Huber M, Berg T, Hinrichsen H, Rasenack J, Heintges T, et al. Peginterferon- alpha-2a (40KD) and ribavirin for 16 or 24 weeks in patients with genotype 2 or 3 chronic hepatitis C. Gastroenterology 2005;129:522-527.


267. Muir AJ, Bornstein JD, Killenberg PG. Atlantic Coast Hepatitis Treatment Group. Peginterferon alfa-2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-Hispanic whites. N Engl J Med 2004;350:2265-2271.


268. Dai CY, Huang JF, Hsieh MY, Hou NJ, Lin ZY, Chen SC, et al. Insulin resistance predicts response to PegIFN-a-alpha/ribavirin combination therapy in chronic hepatitis C patients. J Hepatol 2009;50:712-718.


269. Romero-Gomez M, Del Mar Viloria M, Andrade RJ, Salmeron J, Diago M, Fernandez-Rodriguez CM, et al. Insulin resistance impairs sustained response rate to PegIFN-a plus ribavirin in chronic hepatitis C patients. Gastroenterology 2005;128:636-641.


270. Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, et al. Genome- wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet 2009;41:1105-1109.


271. Mangia A, Thompson AJ, Santoro R, Piazzolla V, Tillmann HL, Patel K, et al. An IL28B polymorphism determines treatment response of hepatitis C virus genotype 2 or 3 patients who do not achieve a rapid virologic response. Gastroenterology 2010;139:821-827 827. e1.


272. Lyoo K, Song MJ, Hur W, Choi JE, Hong SW, Kim CW, et al. Polymorphism near the IL28B gene in Korean hepatitis C virus-infected patients treated with peg-interferon plus ribavirin. J Clin Virol 2011;52:363-366.


274. Jung YK, Kim JH, Ahn SM, Yang JW, Park SJ, Kim JW, et al. Role of interleukin 28B-related gene polymorphisms in chronic hepatitis C and the response to antiviral therapy in Koreans. J Clin Gastroenterol 2013;47:644-650.


275. McHutchison JG, Manns M, Patel K, Poynard T, Lindsay KL, Trepo C, et al. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology 2002;123:1061-1069.


276. Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014;370:1889-1898.


277. Alqahtani SA, Afdhal N, Zeuzem S, Gordon SC, Mangia A, Kwo P, et al. Safety and tolerability of ledipasvir/sofosbuvir with and without ribavirin in patients with chronic hepatitis C virus genotype 1 infection: Analysis of phase III ION trials. Hepatology 2015;62:25-30.


278. Jacobson IM, Kwo PY, Kowdley KV, Yang JC, Zhu Y, Hyland RH, et al. Virologic response rates to all oral fixed-dose combination ledipasvir/sofosbuvir regimens are similar in patients with and without traditional negative predictive factors in phase 3 clinical trials [Abstract]. Hepatology 2014;60:1141A-1142A.
279. Bourliere M, Sulkowski MS, Omata M, Zeuzem S, Feld JJ, Lawitz E, et al. An integrated safety and efficacy analysis of ’╝×500 patients with compensated cirrhosis treated with ledipasvir/sofosbuvir with or without ribavirin [Abstract]. Hepatology 2014;60:239A.

280. Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med 2014;370:1594-1603.


281. Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med 2014;370:1983-1992.


282. Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med 2014;370:1973-1982.


283. Feld J, Moreno C, Trinh R, Tam E, Bourgeois S, Horsmans Y, et al. Turquoise-III: safety and efficacy of 12-week ribavirin-free treatment for patients with HCV genotype 1b and cirrhosis [Abstract]. J Viral Hepat 2015;22:135A.
284. Kao JH, Peng CY, Chang TT, Heo J, Chu CJ, Lee YJ, et al. All-oral dual Therapy with daclatasvir and asunaprevir in patients in Korea and Taiwan with HCV genotype 1b infection [Abstract]. Hepatol Int 2015;9:74A-75A.
285. Lawitz E, Sulkowski MS, Ghalib R, Rodriguez-Torres M, Younossi ZM, Corregidor A, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet 2014;384:1756-1765.


286. Kwo P, Gitlin N, Nahass R, Bernstein D, Rojter S, Schiff E, et al. A Phase 3, randomised, open-label study to evaluate the efficacy and safety of 12 and 8 weeks of simeprevir (SMV) plus sofosbuvir (SOF) in treatment-naïve and-experienced patients with chronic HCV genotype 1 infection without cirrhosis: OPTIMIST-1 [Abstract]. J Hepatol 2015;62:270A.

287. Lawitz E, Matusow G, De Jesus E, Yoshida E, Felizarta F, Ghalib R, et al. A phase 3, open-label, single-arm study to evaluate the efficacy and safety of 12 weeks of simeprevir (SMV) plus sofosbuvir (SOF) in treatment-naïve or-experienced patients with chronic HCV genotype 1 infection and cirrhosis: Optimist-2 [Abstract]. J Hepatol 2015;62:264A-265A.

288. Dieterich D, Bacon BR, Flamm SL, Kowdley KV, Milligan S, Tsai N, et al. Evaluation of sofosbuvir and simeprevir-based regimens in the TRIO network: academic and community treatment of a real-world, heterogeneous population [Abstract]. Hepatology 2014;60:220A.
289. Kowdley KV, Lawitz E, Crespo I, Hassanein T, Davis MN, DeMicco M, et al. Sofosbuvir with pegylated interferon alfa-2a and ribavirin for treatment-naive patients with hepatitis C genotype-1 infection (ATOMIC): an open-label, randomised, multicentre phase 2 trial. Lancet 2013;381:2100-2107.


290. Jacobson IM, Dore GJ, Foster GR, Fried MW, Radu M, Rafalsky VV, et al. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet 2014;384:403-413.


291. Manns M, Marcellin P, Poordad F, de Araujo ES, Buti M, Horsmans Y, et al. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomised, double-blind, placebo- controlled phase 3 trial. Lancet 2014;384:414-426.


292. Afdhal NH, McHutchison JG, Zeuzem S, Mangia A, Pawlotsky JM, Murray JS, et al. Hepatitis C pharmacogenetics: state of the art in 2010. Hepatology 2011;53:336-345.


293. Jeong SW, Kim JD, Woo HY, You CR, Lee SW, Song MJ, et al. Impact of adherence to PegIFN-a-ribavirin combination therapy in chronic hepatitis C patients on achieving a sustained virologic response. Korean J Hepatol 2009;15:338-349.


294. Kang MJ, Jung EU, Park SW, Choi P, Kim JH, Park SJ, et al. Effects of pegylated interferon and ribavirin in Korean patients with chronic hepatitis C virus infection. Korean J Hepatol 2008;14:318-330.


297. Sinn DH, Shin SR, Kil JS, Kim J, Gwak GY, Choi MS, et al. Efficacy of peg-interferon-alpha-2a plus ribavirin for patients aged 60 years and older with chronic hepatitis C in Korea. J Gastroenterol Hepatol 2011;26:469-476.


298. Park SY, Rim MY, Yo IK, Ha MS, Kim JS, Lee JW, et al. Efficacy of PegIFN-a and ribavirin combination therapy of chronic hepatitis C: a pooled analysis. Korean J Gastroenterol 2012;60:306-314.


299. Berg T, Sarrazin C, Herrmann E, Hinrichsen H, Gerlach T, Zachoval R, et al. Prediction of treatment outcome in patients with chronic hepatitis C: significance of baseline parameters and viral dynamics during therapy. Hepatology 2003;37:600-609.


300. Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 2014;370:1483-1493.


301. Ahn SH, Jeong S, Paik S, Yang J, Mo H, Ga B, et al. Korean patients with genotype 1 and 2 HCV infection achieved over 97% sustained virologic response following 12 weeks of ledipasvir/sofosbuvir or sofosbuvir plus ribavirin [Abstract]. Hepatol Int 2015;9:S72.
302. Mizokami M, Yokosuka O, Takehara T, Sakamoto N, Korenaga M, Mochizuki H, et al. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, phase 3 trial. Lancet Infect Dis 2015;15:645-653.


303. Bourli├©re M, Bronowicki JP, de Ledinghen V, H├®zode C, Zoulim F, Mathurin P, et al. Ledipasvir-sofosbuvir with or without ribavirin to treat patients with HCV genotype 1 infection and cirrhosis non-responsive to previous protease-inhibitor therapy: a randomised, double-blind, phase 2 trial (SIRIUS). Lancet Infect Dis 2015;15:397-404.


304. Zeuzem S, Jacobson IM, Baykal T, Marinho RT, Poordad F, Bourli├©re M, et al. Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med 2014;370:1604-1614.


305. Andreone P, Colombo MG, Enejosa JV, Koksal I, Ferenci P, Maieron A, et al. ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology 2014;147:359-365 e1.


307. Pol S, Bourliere M, Lucier S, De Ledinghen V, Zoulim F, Dorival-Mouly C, et al. Safety and efficacy of the combination daclatasvirsofosbuvir in HCV genotype 1-mono-infected patients from the French observational cohort ANRS CO22 HEPATHER [Abstract]. J Hepatol 2015;62(Suppl 2):S258.

308. Reddy KR, Zeuzem S, Zoulim F, Weiland O, Horban A, Stanciu C, et al. Simeprevir versus telaprevir with PegIFN-a and ribavirin in previous null or partial responders with chronic hepatitis C virus genotype 1 infection (ATTAIN): a randomised, double-blind, non-inferiority phase 3 trial. Lancet Infect Dis 2015;15:27-35.


309. Kao JH, Ahn SH, Chien RN, Jeong SH, Peng CY, Lim YS, et al. P0809: 98% SVR12 in Korean and Taiwanese patients with chronic genotype 2 HCV infection receiving 12 weeks of sofosbuvir plus ribavirin: results from an international, multicenter phase 3 study. J Hepatol 2015;62:S638.

310. Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med 2013;368:1867-1877.


311. Wyles DL, Ruane PJ, Sulkowski MS, Dieterich D, Luetkemeyer A, Morgan TR, et al. Daclatasvir plus Sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med 2015;373:714-725.


312. Jacobson IM, Brown RS Jr, Freilich B, Afdhal N, Kwo PY, Santoro J, et al. Peginterferon alfa-2b and weight-based or flat-dose ribavirin in chronic hepatitis C patients: a randomized trial. Hepatology 2007;46:971-981.


313. Zeuzem S, Hultcrantz R, Bourliere M, Goeser T, Marcellin P, Sanchez-Tapias J, et al. Peginterferon alfa-2b plus ribavirin for treatment of chronic hepatitis C in previously untreated patients infected with HCV genotypes 2 or 3. J Hepatol 2004;40:993-999.


314. Ferenci P, Brunner H, Laferl H, Scherzer TM, Maieron A, Strasser M, et al. A randomized, prospective trial of ribavirin 400 mg/day versus 800 mg/day in combination with PegIFN-a alfa-2a in hepatitis C virus genotypes 2 and 3. Hepatology 2008;47:1816-1823.


315. Lee S, Kim IH, Kim SH, Kim SW, Lee SO, Lee ST, et al. Efficacy and tolerability of pegylated interferon-alpha2a plus ribavirin versus pegylated interferon-alpha2b plus ribavirin in treatment-naive chronic hepatitis C patients. Intervirology 2010;53:146-153.


316. Diago M, Shiffman ML, Bronowicki JP, Zeuzem S, Rodriguez-Torres M, Pappas SC, et al. Identifying hepatitis C virus genotype 2/3 patients who can receive a 16-week abbreviated course of PegIFN-a alfa-2a (40KD) plus ribavirin. Hepatology 2010;51:1897-1903.


317. Lagging M, Langeland N, Pedersen C, F├żrkkil├ż M, Buw MR, M├Ėrch K, et al. Randomized comparison of 12 or 24 weeks of PegIFN-a-2a and ribavirin in chronic hepatitis C virus genotype 2/3 infection. Hepatology 2008;47:1837-1845.


318. Poynard T, Munteanu M, Colombo M, Bruix J, Schiff E, Terg R, et al. FibroTest is an independent predictor of virologic response in chronic hepatitis C patients retreated with pegylated interferon alfa-2b and ribavirin in the EPIC(3) program. J Hepatol 2011;54:227-235.


319. Mangia A, Santoro R, Minerva N, Ricci GL, Carretta V, Persico M, et al. Peginterferon alfa-2b and ribavirin for 12 vs. 24 weeks in HCV genotype 2 or 3. N Engl J Med 2005;352:2609-2617.


320. Shiffman ML, Suter F, Bacon BR, Nelson D, Harley H, Sol├Ī R, et al. Peginterferon alfa-2a and ribavirin for 16 or 24 weeks in HCV genotype 2 or 3. N Engl J Med 2007;357:124-134.


321. Aghemo A, Rumi MG, Monico S, Prati GM, DŌĆÖAmbrosio R, Donato MF, et al. The pattern of pegylated interferon-alpha2b and ribavirin treatment failure in cirrhotic patients depends on hepatitis C virus genotype. Antivir Ther 2009;14:577-584.

322. Marciano S, Gadano AC. How to optimize current treatment of genotype 2 hepatitis C virus infection. Liver Int 2014;34 Suppl 1:13-17.


323. Marcellin P, Cheinquer H, Curescu M, Dusheiko GM, Ferenci P, Horban A, et al. High sustained virologic response rates in rapid virologic response patients in the large real-world PROPHESYS cohort confirm results from randomized clinical trials. Hepatology 2012;56:2039-2050.


325. Dalgard O, Bj├Ėro K, Ring-Larsen H, Bjornsson E, Holberg-Petersen M, Skovlund E, et al. Pegylated interferon alfa and ribavirin for 14 versus 24 weeks in patients with hepatitis C virus genotype 2 or 3 and rapid virological response. Hepatology 2008;47:35-42.


326. Mecenate F, Barbaro G, Pellicelli A, Barlattani A, Mazzoni E, Bonaventura ME, et al. Comparison of peg-interferon alfa-2a and ribavirin for 12 or 24 weeks in patients with HCV genotype 2 or 3: The cleo trial. Hepatology 2007;46:828A.
327. Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med 2014;370:1993-2001.


328. Omata M, Nishiguchi S, Ueno Y, Mochizuki H, Izumi N, Ikeda F, et al. 97% Sustained virologic response in Japanese patients with chronic genotype 2 hepatitis C virus infection receiving sofosbuvir in combination with ribavirin for 12 weeks: results from a phase 3 multicenter study. Hepatology 2014;60:671A.

329. Shiffman ML, Di Bisceglie AM, Lindsay KL, Morishima C, Wright EC, Everson GT, et al. Peginterferon alfa-2a and ribavirin in patients with chronic hepatitis C who have failed prior treatment. Gastroenterology 2004;126:1015-1023 discussion 947.


330. Jacobson IM, Gonzalez SA, Ahmed F, Lebovics E, Min AD, Bodenheimer HC Jr, et al. A randomized trial of pegylated interferon alpha-2b plus ribavirin in the retreatment of chronic hepatitis C. Am J Gastroenterol 2005;100:2453-2462.


331. Taliani G, Gemignani G, Ferrari C, Aceti A, Bartolozzi D, Blanc PL, et al. Pegylated interferon alfa-2b plus ribavirin in the retreatment of interferon-ribavirin nonresponder patients. Gastroenterology 2006;130:1098-1106.


334. Sagir A, Heintges T, Akyazi Z, Oette M, Erhardt A, H├żussinger D. Relapse to prior therapy is the most important factor for the retreatment response in patients with chronic hepatitis C virus infection. Liver Int 2007;27:954-959.


335. Krawitt EL, Ashikaga T, Gordon SR, Ferrentino N, Ray MA, Lidofsky SD, et al. Peginterferon alfa-2b and ribavirin for treatment-refractory chronic hepatitis C. J Hepatol 2005;43:243-249.


336. Cheruvattath R, Rosati MJ, Gautam M, Vargas HE, Rakela J, Balan V. Pegylated interferon and ribavirin failures: is retreatment an option? Dig Dis Sci 2007;52:732-736.


337. Foster GR, Pianko S, Cooper C, Brown A, Forton D, Nahass RG. Sofosbuvir plus Peg-IFN/ribavirin for 12 weeks vs sofosbuvir/ribavirin for 16 or 24 weeks in genotype 3 HCV-infected patients and treatment-experienced cirrhotic patients with genotype 2 HCV: the Boson Study. J Hepatol 2015;62:S259-S260.

339. Hezode C, De Ledinghen V, Fontaine H, Zoulim F, Lebray P, Boyer N, et al. LP05: Daclatasvir plus sofosbuvir with or without ribavirin in patients with HCV genotype 3 infection: interim analysis of a French multicenter compassionate use program. J Hepatol 2015;62:S265-S266.

340. Gane EJ, Hyland RH, An D, Svarovskaia E, Pang PS, Brainard D, et al. Efficacy of ledipasvir and sofosbuvir, with or without ribavirin, for 12 weeks in patients with HCV genotype 3 or 6 infection. Gastroenterology 2015;149:1454-1461 e1.


341. Lawitz E, Lalezari JP, Hassanein T, Kowdley KV, Poordad FF, Sheikh AM, et al. Sofosbuvir in combination with PegIFN-a alfa-2a and ribavirin for non-cirrhotic, treatment-naive patients with genotypes 1, 2, and 3 hepatitis C infection: a randomised, double-blind, phase 2 trial. Lancet Infect Dis 2013;13:401-408.


342. Gane EJ, Stedman CA, Hyland RH, Ding X, Svarovskaia E, Symonds WT, et al. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med 2013;368:34-44.


344. Poynard T, Colombo M, Bruix J, Schiff E, Terg R, Flamm S, et al. Peginterferon alfa-2b and ribavirin: effective in patients with hepatitis C who failed interferon alfa/ribavirin therapy. Gastroenterology 2009;136:1618-1628 e2.


345. Alfaleh FZ, Hadad Q, Khuroo MS, Aljumah A, Algamedi A, Alashgar H, et al. Peginterferon alpha-2b plus ribavirin compared with interferon alpha-2b plus ribavirin for initial treatment of chronic hepatitis C in Saudi patients commonly infected with genotype 4. Liver Int 2004;24:568-574.


346. Ruane PJ, Ain D, Stryker R, Meshrekey R, Soliman M, Wolfe PR, et al. Sofosbuvir plus ribavirin for the treatment of chronic genotype 4 hepatitis C virus infection in patients of Egyptian ancestry. J Hepatol 2015;62:1040-1046.


347. Doss W, Shiha G, Hassany M, Soliman R, Fouad R, Khairy M, et al. Sofosbuvir plus ribavirin for treating Egyptian patients with hepatitis C genotype 4. J Hepatol 2015;63:581-585.


348. Molina JM, Orkin C, Iser DM, Zamora FX, Nelson M, Stephan C, et al. Sofosbuvir plus ribavirin for treatment of hepatitis C virus in patients co-infected with HIV (PHOTON-2): a multicentre, open-label, non-randomised, phase 3 study. Lancet 2015;385:1098-1106.


350. H├®zode C, Asselah T, Reddy KR, Hassanein T, Berenguer M, Fleischer-Stepniewska K, et al. Ombitasvir plus paritaprevir plus ritonavir with or without ribavirin in treatment-naive and treatment-experienced patients with genotype 4 chronic hepatitis C virus infection (PEARL-I): a randomised, open-label trial. Lancet 2015;385:2502-2509.


351. Moreno C, Hezode C, Marcellin P, Bourgeois S, Francque S, Samuel D, et al. Efficacy and safety of simeprevir with PegIFN/ribavirin in naïve or experienced patients infected with chronic HCV genotype 4. J Hepatol 2015;62:1047-1055.


352. Nguyen MH, Keeffe EB. Chronic hepatitis C: genotypes 4 to 9. Clin Liver Dis 2005;9:411-426 vi.


353. Yuen MF, Lai CL. Response to combined interferon and ribavirin is better in patients infected with hepatitis C virus genotype 6 than genotype 1 in Hong Kong. Intervirology 2006;49:96-98.


354. Tsang OT, Zee JS, Chan JM, Li RS, Kan YM, Li FT, et al. Chronic hepatitis C genotype 6 responds better to pegylated interferon and ribavirin combination therapy than genotype 1. J Gastroenterol Hepatol 2010;25:766-771.


355. Nguyen NH, VuTien P, Garcia RT, Trinh H, Nguyen H, Nguyen K, et al. Response to pegylated interferon and ribavirin in Asian American patients with chronic hepatitis C genotypes 1 vs 2/3 vs 6. J Viral Hepat 2010;17:691-697.


356. Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS Jr, et al. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology 2015;149:649-659.


357. Poordad F, Schiff ER, Vierling JM, Landis C, Fontana RJ, Yang R, et al. LO8 : Daclatasvir, sofosbuvir, and ribavirin combination for HCV patients with advanced cirrhosis or posttransplant recurrence: phase 3 ALLY-1 study. J Hepatol 2015;62:Suppl-261 -S262.

359. Cheong HR, Woo HY, Heo J, Yoon KT, Kim DU, Kim GH, et al. Clinical efficacy and safety of the combination therapy of PegIFN-a and ribavirin in cirrhotic patients with HCV infection. Korean J Hepatol 2010;16:38-48.


360. Garcia-Retortillo M, Forns X, Feliu A, Moitinho E, Costa J, Navasa M, et al. Hepatitis C virus kinetics during and immediately after liver transplantation. Hepatology 2002;35:680-687.


361. Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucey MR. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology 2002;122:889-896.


362. Prieto M, Berenguer M, Ray├│n JM, C├│rdoba J, Arg├╝ello L, Carrasco D, et al. High incidence of allograft cirrhosis in hepatitis C virus genotype 1b infection following transplantation: relationship with rejection episodes. Hepatology 1999;29:250-256.


363. Gane E, Pilmore H. Management of chronic viral hepatitis before and after renal transplantation. Transplantation 2002;74:427-437.


364. Curry MP, Forns X, Chung RT, Terrault NA, Brown R Jr, Fenkel JM, et al. Sofosbuvir and ribavirin prevent recurrence of HCV infection after liver transplantation: an open-label study. Gastroenterology 2015;148:100-107 e1.


365. Pungpapong S, Aqel B, Leise M, Werner KT, Murphy JL, Henry TM, et al. Multicenter experience using simeprevir and sofosbuvir with or without ribavirin to treat hepatitis C genotype 1 after liver transplant. Hepatology 2015;61:1880-1886.


366. Kwo PY, Mantry PS, Coakley E, Te HS, Vargas HE, Brown R Jr, et al. An interferon-free antiviral regimen for HCV after liver transplantation. N Engl J Med 2014;371:2375-2382.


367. Samuel D, Bizollon T, Feray C, Roche B, Ahmed SN, Lemonnier C, et al. Interferon-alpha 2b plus ribavirin in patients with chronic hepatitis C after liver transplantation: a randomized study. Gastroenterology 2003;124:642-650.


368. Carri├│n JA, Navasa M, Garc├Ła-Retortillo M, Garc├Ła-Pagan JC, Crespo G, Bruguera M, et al. Efficacy of antiviral therapy on hepatitis C recurrence after liver transplantation: a randomized controlled study. Gastroenterology 2007;132:1746-1756.


369. Angelico M, Petrolati A, Lionetti R, Lenci I, Burra P, Donato MF, et al. A randomized study on Peg-interferon alfa-2a with or without ribavirin in liver transplant recipients with recurrent hepatitis C. J Hepatol 2007;46:1009-1017.


370. Martin P, Fabrizi F. Hepatitis C virus and kidney disease. J Hepatol 2008;49:613-624.


371. Bonacci M, Londo├▒o MC, Esforzado N, Forns X, Sotoca JM, Campistol JM. Antiviral treatment with sofosbuvir and simeprevir in a kidney transplant recipient with HCV-decompensated cirrhosis: viral eradication and removal from the liver transplant waiting list. Transpl Int 2015;28:1345-1349.


372. Huard G, Kim B, Patel A, Aljarallah B, Perumalswami P, Odin JA, et al. Early safety and efficacy profiles of renal transplant recipients with chronic hepatitis C treated with Sofosbuvir and Ribavirin. Hepatology 2014;60(S1):540A-541A.
373. Licata A, Di Bona D, Schepis F, Shahied L, Crax├Ł A, Camm├Ā C. When and how to treat acute hepatitis C? J Hepatol 2003;39:1056-1062.


374. Wang CC, Krantz E, Klarquist J, Krows M, McBride L, Scott EP, et al. Acute hepatitis c in a contemporary US cohort: modes of acquisition and factors influencing viral clearance. J Infect Dis 2007;196:1474-1482.


376. Kamal SM, Fouly AE, Kamel RR, Hockenjos B, Al Tawil A, Khalifa KE, et al. Peginterferon alfa-2b therapy in acute hepatitis C: impact of onset of therapy on sustained virologic response. Gastroenterology 2006;130:632-638.


377. Deuffic-Burban S, Castel H, Wiegand J, Manns MP, Wedemeyer H, Mathurin P, et al. Immediate vs. delayed treatment in patients with acute hepatitis C based on IL28B polymorphism: a model-based analysis. J Hepatol 2012;57:260-266.


378. Deterding K, Gr├╝ner N, Buggisch P, Wiegand J, Galle PR, Spengler U, et al. Delayed versus immediate treatment for patients with acute hepatitis C: a randomised controlled non-inferiority trial. Lancet Infect Dis 2013;13:497-506.


379. Jaeckel E, Cornberg M, Wedemeyer H, Santantonio T, Mayer J, Zankel M, et al. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med 2001;345:1452-1457.


380. Nomura H, Sou S, Tanimoto H, Nagahama T, Kimura Y, Hayashi J, et al. Short-term interferon-alfa therapy for acute hepatitis C: a randomized controlled trial. Hepatology 2004;39:1213-1219.


381. Kamal SM, Ismail A, Graham CS, He Q, Rasenack JW, Peters T, et al. Pegylated interferon alpha therapy in acute hepatitis C: relation to hepatitis C virus-specific T cell response kinetics. Hepatology 2004;39:1721-1731.


382. Broers B, Helbling B, Fran├¦ois A, Schmid P, Chuard C, Hadengue A, et al. Barriers to interferon-alpha therapy are higher in intravenous drug users than in other patients with acute hepatitis C. J Hepatol 2005;42:323-328.


383. Santantonio T, Fasano M, Sinisi E, Guastadisegni A, Casalino C, Mazzola M, et al. Efficacy of a 24-week course of PEG-interferon alpha-2b monotherapy in patients with acute hepatitis C after failure of spontaneous clearance. J Hepatol 2005;42:329-333.


384. Wiegand J, Buggisch P, Boecher W, Zeuzem S, Gelbmann CM, Berg T, et al. Early monotherapy with pegylated interferon alpha-2b for acute hepatitis C infection: the HEP-NET acute-HCV-II study. Hepatology 2006;43:250-256.


385. Kamal SM, Moustafa KN, Chen J, Fehr J, Abdel Moneim A, Khalifa KE, et al. Duration of PegIFN-a therapy in acute hepatitis C: a randomized trial. Hepatology 2006;43:923-931.


387. Santantonio T, Fasano M, Sagnelli E, Tundo P, Babudieri S, Fabris P, et al. Acute hepatitis C: a 24-week course of pegylated interferon ╬▒-2b versus a 12-week course of pegylated interferon ╬▒-2b alone or with ribavirin. Hepatology 2014;59:2101-2109.


388. Sublette VA, Douglas MW, McCaffery K, George J, Perry KN. Psychological, lifestyle and social predictors of hepatitis C treatment response: a systematic review. Liver Int 2013;33:894-903.


389. Mishra P, Florian J, Qi K, Zeng W, Naeger LK, Donaldson E, et al. FDA perspective on sofosbuvir therapy for patients with chronic hepatitis C virus genotype 1 infection who did not respond to treatment with pegylated interferon and ribavirin. Gastroenterology 2014;147:1196-1200.


390. Pol S, Sulkowski MS, Hassanein T, Gane EJ, Liu L, Mo H, et al. Sofosbuvir plus pegylated interferon and ribavirin in patients with genotype 1 hepatitis C virus in whom previous therapy with direct-acting antivirals has failed. Hepatology 2015;62:129-134.


392. AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 2015;62:932-954.


393. Backus LI, Boothroyd DB, Phillips BR, Belperio P, Halloran J, Mole LA. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol 2011;9:509-516 e1.


395. Dalgard O. Follow-up studies of treatment for hepatitis C virus infection among injection drug users. Clin Infect Dis 2005;40 Suppl 5:S336-S338.

396. Currie SL, Ryan JC, Tracy D, Wright TL, George S, McQuaid R, et al. A prospective study to examine persistent HCV reinfection in injection drug users who have previously cleared the virus. Drug Alcohol Depend 2008;93:148-154.


397. Grebely J, Knight E, Ngai T, Genoway KA, Raffa JD, Storms M, et al. Reinfection with hepatitis C virus following sustained virological response in injection drug users. J Gastroenterol Hepatol 2010;25:1281-1284.


399. Th├®venot T, Cadranel JF, Di Martino V, Pariente A, Causse X, Renou C, et al. A national French survey on the use of growth factors as adjuvant treatment of chronic hepatitis C. Hepatology 2007;45:377-383.


400. McHutchison JG, Dusheiko G, Shiffman ML, Rodriguez-Torres M, Sigal S, Bourliere M, et al. Eltrombopag for thrombocytopenia in patients with cirrhosis associated with hepatitis C. N Engl J Med 2007;357:2227-2236.


401. Afdhal NH, Giannini EG, Tayyab G, Mohsin A, Lee JW, Andriulli A, et al. Eltrombopag before procedures in patients with cirrhosis and thrombocytopenia. N Engl J Med 2012;367:716-724.


402. Janssen HL, Brouwer JT, van der Mast RC, Schalm SW. Suicide associated with alfa-interferon therapy for chronic viral hepatitis. J Hepatol 1994;21:241-243.


403. Udina M, Castellv├Ł P, Moreno-Espa├▒a J, Navin├®s R, Vald├®s M, Forns X, et al. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry 2012;73:1128-1138.


404. Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, et al. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med 2001;344:961-966.


405. de Knegt RJ, Bezemer G, Van Gool AR, Drenth JP, Hansen BE, Droogleever Fortuyn HA, et al. Randomised clinical trial: escitalopram for the prevention of psychiatric adverse events during treatment with PegIFN-a-alfa-2a and ribavirin for chronic hepatitis C. Aliment Pharmacol Ther 2011;34:1306-1317.


407. Mandac JC, Chaudhry S, Sherman KE, Tomer Y. The clinical and physiological spectrum of interferon-alpha induced thyroiditis: toward a new classification. Hepatology 2006;43:661-672.


408. Marazuela M, Garc├Ła-Buey L, Gonz├Īlez-Fern├Īndez B, Garc├Ła-Monz├│n C, Arranz A, Borque MJ, et al. Thyroid autoimmune disorders in patients with chronic hepatitis C before and during interferon-alpha therapy. Clin Endocrinol (Oxf) 1996;44:635-642.


409. Lisker-Melman M, Di Bisceglie AM, Usala SJ, Weintraub B, Murray LM, Hoofnagle JH. Development of thyroid disease during therapy of chronic viral hepatitis with interferon alfa. Gastroenterology 1992;102:2155-2160.


410. Martocchia A, Labbadia G, Paoletti V, Gargano S, Grossi A, Trabace S, et al. HashimotoŌĆÖs disease during interferon-alpha therapy in a patient with pre-treatment negative anti-thyroid autoantibodies and with the specific genetic susceptibility to the thyroid disease. Neuro Endocrinol Lett 2001;22:49-52.

411. Carella C, Mazziotti G, Morisco F, Manganella G, Rotondi M, Tuccillo C, et al. Long-term outcome of interferon-alpha-induced thyroid autoimmunity and prognostic influence of thyroid autoantibody pattern at the end of treatment. J Clin Endocrinol Metab 2001;86:1925-1929.

412. Wong V, Fu AX, George J, Cheung NW. Thyrotoxicosis induced by alpha-interferon therapy in chronic viral hepatitis. Clin Endocrinol (Oxf) 2002;56:793-798.


413. Gutkowski K, Gutkowska D, Bilkiewicz T. Interferon therapy in chronic viral hepatitis; an autoimmunity dilemma. Przegl Lek 2007;64:148-152.

414. Panetta JD, Gilani N. Interferon-induced retinopathy and its risk in patients with diabetes and hypertension undergoing treatment for chronic hepatitis C virus infection. Aliment Pharmacol Ther 2009;30:597-602.


415. Malik NN, Sheth HG, Ackerman N, Davies N, Mitchell SM. A prospective study of change in visual function in patients treated with pegylated interferon alpha for hepatitis C in the UK. Br J Ophthalmol 2008;92:256-258.


416. Chisholm JA, Williams G, Spence E, Parks S, Keating D, Gavin M, et al. Retinal toxicity during pegylated alpha-interferon therapy for chronic hepatitis C: a multifocal electroretinogram investigation. Aliment Pharmacol Ther 2005;21:723-732.


418. Vujosevic S, Tempesta D, Noventa F, Midena E, Sebastiani G. Pegylated interferon-associated retinopathy is frequent in hepatitis C virus patients with hypertension and justifies ophthalmologic screening. Hepatology 2012;56:455-463.


420. Schulman JA, Liang C, Kooragayala LM, King J. Posterior segment complications in patients with hepatitis C treated with interferon and ribavirin. Ophthalmology 2003;110:437-442.


421. Formann E, Stauber R, Denk DM, Jessner W, Zollner G, Munda-Steindl P, et al. Sudden hearing loss in patients with chronic hepatitis C treated with pegylated interferon/ribavirin. Am J Gastroenterol 2004;99:873-877.


422. De Franceschi L, Fattovich G, Turrini F, Ayi K, Brugnara C, Manzato F, et al. Hemolytic anemia induced by ribavirin therapy in patients with chronic hepatitis C virus infection: role of membrane oxidative damage. Hepatology 2000;31:997-1004.


423. Afdhal NH, Dieterich DT, Pockros PJ, Schiff ER, Shiffman ML, Sulkowski MS, et al. Epoetin alfa maintains ribavirin dose in HCV-infected patients: a prospective, double-blind, randomized controlled study. Gastroenterology 2004;126:1302-1311.


424. Kochhar DM, Penner JD, Knudsen TB. Embryotoxic, teratogenic, and metabolic effects of ribavirin in mice. Toxicol Appl Pharmacol 1980;52:99-112.


425. Bravo MJ, Vallejo F, Barrio G, Brugal MT, Molist G, Pulido J, et al. HCV seroconversion among never-injecting heroin users at baseline: no predictors identified other than starting injection. Int J Drug Policy 2012;23:415-419.


426. Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med 2006;144:705-714.


427. Kim HS, Choo DH. Prevalence of anti-HCV among drug users in Korea. Korean J Med 1996;50:194-200.
429. European Association for Study of Liver. EASL Recommendations on Treatment of Hepatitis C 2015. J Hepatol 2015;63:199-236.


433. Menon RM, Badri PS, Wang T, Polepally AR, Zha J, Khatri A, et al. Drug-drug interaction profile of the all-oral anti-hepatitis C virus regimen of paritaprevir/ritonavir, ombitasvir, and dasabuvir. J Hepatol 2015;63:20-29.


434. Lalezari J, Sullivan JG, Varunok P, Galen E, Kowdley KV, Rustgi V, et al. Ombitasvir/paritaprevir/r 4and dasabuvir plus ribavirin in HCV genotype 1-infected patients on methadone or buprenorphine. J Hepatol 2015;63:364-369.


435. Liu CH, Kao JH. Treatment of hepatitis C virus infection in patients with end-stage renal disease. J Gastroenterol Hepatol 2011;26:228-239.


436. Bang BK, Choi BS, Kim HW, Kim SK, Yang CW, Kim YS, et al. Retrospective study on the impact of hepatitis B and hepatitis C virus infection on renal transplnat recipients over 15 years. Korean J Nephrol 2002;21:423-434.
437. Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl 2008;(109):S1-99.
438. Marcelli D, Stannard D, Conte F, Held PJ, Locatelli F, Port FK. ESRD patient mortality with adjustment for comorbid conditions in Lombardy (Italy) versus the United States. Kidney Int 1996;50:1013-1018.


439. Maisonneuve P, Agodoa L, Gellert R, Stewart JH, Buccianti G, Lowenfels AB, et al. Cancer in patients on dialysis for end-stage renal disease: an international collaborative study. Lancet 1999;354:93-99.


440. Nakayama E, Akiba T, Marumo F, Sato C. Prognosis of anti-hepatitis C virus antibody-positive patients on regular hemodialysis therapy. J Am Soc Nephrol 2000;11:1896-1902.


441. Bruchfeld A, Wilczek H, Elinder CG. Hepatitis C infection, time in renal-replacement therapy, and outcome after kidney transplantation. Transplantation 2004;78:745-750.


442. Aroldi A, Lampertico P, Montagnino G, Passerini P, Villa M, Campise MR, et al. Natural history of hepatitis B and C in renal allograft recipients. Transplantation 2005;79:1132-1136.


443. Bloom RD, Rao V, Weng F, Grossman RA, Cohen D, Mange KC. Association of hepatitis C with posttransplant diabetes in renal transplant patients on tacrolimus. J Am Soc Nephrol 2002;13:1374-1380.


444. Kamar N, Mariat C, Delahousse M, Dantal J, Al Najjar A, Cassuto E, et al. Diabetes mellitus after kidney transplantation: a French multicentre observational study. Nephrol Dial Transplant 2007;22:1986-1993.


445. Fabrizi F, Martin P, Dixit V, Bunnapradist S, Kanwal F, Dulai G. Post-transplant diabetes mellitus and HCV seropositive status after renal transplantation: meta-analysis of clinical studies. Am J Transplant 2005;5:2433-2440.


446. Roth D, Cirocco R, Zucker K, Ruiz P, Viciana A, Burke G, et al. De novo membranoproliferative glomerulonephritis in hepatitis C virus-infected renal allograft recipients. Transplantation 1995;59:1676-1682.


447. Choy BY, Chan TM, Lai KN. Recurrent glomerulonephritis after kidney transplantation. Am J Transplant 2006;6:2535-2542.


448. Pockros PJ, Reddy KR, Mantry PS, Cohen E, Bennett M, Sulkowski MS, et al. LO1: Safety of ombitasvir/paritaprevir/ritonavir plus dasabuvir for treating HCV GT1 infection in patients with severe renal impairment or end-stage renal disease: The RUBY-I study. [Abstract]. J Hepatol 2015;62(Suppl 2):S257.

449. Bruchfeld A, Lindahl K, Reichard O, Carlsson T, Schvarcz R. Pegylated interferon and ribavirin treatment for hepatitis C in haemodialysis patients. J Viral Hepat 2006;13:316-321.


450. Garini G, Allegri L, Carnevali L, Catellani W, Manganelli P, Buzio C. Interferon-alpha in combination with ribavirin as initial treatment for hepatitis C virus-associated cryoglobulinemic membranoproliferative glomerulonephritis. Am J Kidney Dis 2001;38:E35.


451. Mazzaro C, Zorat F, Caizzi M, Donada C, Di Gennaro G, Maso LD, et al. Treatment with peg-interferon alfa-2b and ribavirin of hepatitis C virus-associated mixed cryoglobulinemia: a pilot study. J Hepatol 2005;42:632-638.


452. Saadoun D, Resche-Rigon M, Thibault V, Piette JC, Cacoub P. Antiviral therapy for hepatitis C virus--associated mixed cryoglobulinemia vasculitis: a long-term followup study. Arthritis Rheum 2006;54:3696-3706.


454. Bonacini M, Lin HJ, Hollinger FB. Effect of coexisting HIV-1 infection on the diagnosis and evaluation of hepatitis C virus. J Acquir Immune Defic Syndr 2001;26:340-344.


455. Marcellin P, Martinot-Peignoux M, Elias A, Branger M, Courtois F, Level R, et al. Hepatitis C virus (HCV) viremia in human immunodeficiency virus-seronegative and -seropositive patients with indeterminate HCV recombinant immunoblot assay. J Infect Dis 1994;170:433-435.


456. Thomas DL, Astemborski J, Rai RM, Anania FA, Schaeffer M, Galai N, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA 2000;284:450-456.


457. Goedert JJ, Eyster ME, Lederman MM, Mandalaki T, De Moerloose P, White GC 2nd, et al. End-stage liver disease in persons with hemophilia and transfusion-associated infections. Blood 2002;100:1584-1589.

458. Thomas DL, Shih JW, Alter HJ, Vlahov D, Cohn S, Hoover DR, et al. Effect of human immunodeficiency virus on hepatitis C virus infection among injecting drug users. J Infect Dis 1996;174:690-695.


459. Torriani FJ, Rodriguez-Torres M, Rockstroh JK, Lissen E, Gonzalez-Garc├Ła J, Lazzarin A, et al. Peginterferon Alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med 2004;351:438-450.


460. Bica I, McGovern B, Dhar R, Stone D, McGowan K, Scheib R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis 2001;32:492-497.


461. Palella FJ Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998;338:853-860.


462. Weber R, Sabin CA, Friis-M├Ėller N, Reiss P, El-Sadr WM, Kirk O, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med 2006;166:1632-1641.


463. Qurishi N, Kreuzberg C, L├╝chters G, Effenberger W, Kupfer B, Sauerbruch T, et al. Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus coinfection. Lancet 2003;362:1708-1713.


464. Tien PC. Veterans Affairs Hepatitis C Resource Center Program; National Hepatitis C Program Office. Management and treatment of hepatitis C virus infection in HIV-infected adults: recommendations from the Veterans Affairs Hepatitis C Resource Center Program and National Hepatitis C Program Office. Am J Gastroenterol 2005;100:2338-2354.


466. Sulkowski MS, Mehta SH, Torbenson MS, Higgins Y, Brinkley SC, de Oca RM, et al. Rapid fibrosis progression among HIV/hepatitis C virus-co-infected adults. AIDS 2007;21:2209-2216.


467. Br├żu N, Salvatore M, R├Łos-Bedoya CF, Fern├Īndez-Carbia A, Paronetto F, Rodr├Łguez-Orengo JF, et al. Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol 2006;44:47-55.


468. Mac├Łas J, Berenguer J, Jap├│n MA, Gir├│n JA, Rivero A, L├│pez-Cort├®s LF, et al. Fast fibrosis progression between repeated liver biopsies in patients coinfected with human immunodeficiency virus/hepatitis C virus. Hepatology 2009;50:1056-1063.


469. Opravil M, Sasadeusz J, Cooper DA, Rockstroh JK, Clumeck N, Clotet B, et al. Effect of baseline CD4 cell count on the efficacy and safety of PegIFN-a Alfa-2a (40KD) plus ribavirin in patients with HIV/hepatitis C virus coinfection. J Acquir Immune Defic Syndr 2008;47:36-49.


470. Sulkowski MS. Management of acute and chronic HCV infection in persons with HIV coinfection. J Hepatol 2014;61(1 Suppl):S108-S119.


471. Alvarez D, Dieterich DT, Brau N, Moorehead L, Ball L, Sulkowski MS. Zidovudine use but not weight-based ribavirin dosing impacts anaemia during HCV treatment in HIV-infected persons. J Viral Hepat 2006;13:683-689.


473. Lafeuillade A, Hittinger G, Chadapaud S. Increased mitochondrial toxicity with ribavirin in HIV/HCV coinfection. Lancet 2001;357:280-281.


474. Salmon-C├®ron D, Chauvelot-Moachon L, Abad S, Silbermann B, Sogni P. Mitochondrial toxic effects and ribavirin. Lancet 2001;357:1803-1804.


475. Osinusi A, Townsend K, Kohli A, Nelson A, Seamon C, Meissner EG, et al. Virologic response following combined ledipasvir and sofosbuvir administration in patients with HCV genotype 1 and HIV co-infection. JAMA 2015;313:1232-1239.


476. Sulkowski MS, Eron JJ, Wyles D, Trinh R, Lalezari J, Wang C, et al. Ombitasvir, paritaprevir co-dosed with ritonavir, dasabuvir, and ribavirin for hepatitis C in patients co-infected with HIV-1: a randomized trial. JAMA 2015;313:1223-1231.


479. N├║├▒ez M, Miralles C, Berd├║n MA, Losada E, Aguirrebengoa K, Ocampo A, et al. Role of weight-based ribavirin dosing and extended duration of therapy in chronic hepatitis C in HIV-infected patients: the PRESCO trial. AIDS Res Hum Retroviruses 2007;23:972-982.


480. Ghany MG, Strader DB, Thomas DL, Seeff LB. American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 2009;49:1335-1374.


481. Cargnel A, Angeli E, Mainini A, Gubertini G, Giorgi R, Schiavini M, et al. Open, randomized, multicentre italian trial on PEG-IFN plus ribavirin versus PEG-IFN monotherapy for chronic hepatitis C in HIV-coinfected patients on HAART. Antivir Ther 2005;10:309-317.

482. Santin M, Shaw E, Garcia MJ, Delejido A, de Castro ER, Rota R, et al. Efficacy and safety of pegylated interferon-alpha2b plus ribavirin for the treatment of chronic hepatitis C in HIV-infected patients. AIDS Res Hum Retroviruses 2006;22:315-320.


484. Fern├Īndez-Montero JV, Soriano V. Management of hepatitis C in HIV and/or HBV co-infected patients. Best Pract Res Clin Gastroenterol 2012;26:517-530.


486. Chiaramonte M, Stroffolini T, Vian A, Stazi MA, Floreani A, Lorenzoni U, et al. Rate of incidence of hepatocellular carcinoma in patients with compensated viral cirrhosis. Cancer 1999;85:2132-2137.


487. Lee LP, Dai CY, Chuang WL, Chang WY, Hou NJ, Hsieh MY, et al. Comparison of liver histopathology between chronic hepatitis C patients and chronic hepatitis B and C-coinfected patients. J Gastroenterol Hepatol 2007;22:515-517.


488. Sagnelli E, Pasquale G, Coppola N, Scarano F, Marrocco C, Scolastico C, et al. Influence of chronic coinfection with hepatitis B and C virus on liver histology. Infection 2004;32:144-148.


489. Benvegn├╣ L, Fattovich G, Noventa F, Tremolada F, Chemello L, Cecchetto A, et al. Concurrent hepatitis B and C virus infection and risk of hepatocellular carcinoma in cirrhosis. A prospective study. Cancer 1994;74:2442-2448.


490. Potthoff A, Manns MP, Wedemeyer H. Treatment of HBV/HCV coinfection. Expert Opin Pharmacother 2010;11:919-928.


491. Potthoff A, Wedemeyer H, Boecher WO, Berg T, Zeuzem S, Arnold J, et al. The HEP-NET B/C co-infection trial: a prospective multicenter study to investigate the efficacy of pegylated interferon-alpha2b and ribavirin in patients with HBV/HCV co-infection. J Hepatol 2008;49:688-694.


492. Liu CJ, Chuang WL, Lee CM, Yu ML, Lu SN, Wu SS, et al. Peginterferon alfa-2a plus ribavirin for the treatment of dual chronic infection with hepatitis B and C viruses. Gastroenterology 2009;136:496-504 e3.


493. Collins JM, Raphael KL, Terry C, Cartwright EJ, Pillai A, Anania FA, et al. Hepatitis B virus reactivation during successful treatment of Hepatitis C virus with Sofosbuvir and Simeprevir. Clin Infect Dis 2015;61:1304-1306.


494. Potthoff A, Berg T, Wedemeyer H. HEP-NET B/C Coinfection Study Group. Late hepatitis B virus relapse in patients co-infected with hepatitis B virus and hepatitis C virus after antiviral treatment with pegylated interferon-a2b and ribavirin. Scand J Gastroenterol 2009;44:1487-1490.


495. Asian Pacific Association for the Study of the Liver (APASL) Hepatitis C Working Party, McCaughan GW, Omata M, Amarapurkar D, Bowden S, Chow WC, et al. Asian Pacific Association for the Study of the Liver consensus statements on the diagnosis, management and treatment of hepatitis C virus infection. J Gastroenterol Hepatol 2007;22:615-633.


496. Vento S, Cainelli F, Cesario F. Infections and thalassaemia. Lancet Infect Dis 2006;6:226-233.


497. Plug I, Van Der Bom JG, Peters M, Mauser-Bunschoten EP, De Goede-Bolder A, Heijnen L, et al. Mortality and causes of death in patients with hemophilia, 1992-2001: a prospective cohort study. J Thromb Haemost 2006;4:510-516.


499. Maor Y, Bashari D, Kenet G, Lubetsky A, Luboshitz J, Schapiro JM, et al. Non-invasive biomarkers of liver fibrosis in haemophilia patients with hepatitis C: can you avoid liver biopsy? Haemophilia 2006;12:372-379.


500. Honda T, Katano Y, Kuzuya T, Hayashi K, Ishigami M, Itoh A, et al. Comparison of the efficacy of ribavirin plus PegIFN-a alfa-2b for chronic hepatitis C infection in patients with and without coagulation disorders. J Med Virol 2013;85:228-234.


502. Ozguroglu M, Bilici A, Turna H, Serdengecti S. Reactivation of hepatitis B virus infection with cytotoxic therapy in non-HodgkinŌĆÖs lymphoma. Med Oncol 2004;21:67-72.


503. Yeo W, Chan PK, Zhong S, Ho WM, Steinberg JL, Tam JS, et al. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: a prospective study of 626 patients with identification of risk factors. J Med Virol 2000;62:299-307.


504. Kawatani T, Suou T, Tajima F, Ishiga K, Omura H, Endo A, et al. Incidence of hepatitis virus infection and severe liver dysfunction in patients receiving chemotherapy for hematologic malignancies. Eur J Haematol 2001;67:45-50.


505. Markovic S, Drozina G, Vovk M, Fidler-Jenko M. Reactivation of hepatitis B but not hepatitis C in patients with malignant lymphoma and immunosuppressive therapy. A prospective study in 305 patients. Hepatogastroenterology 1999;46:2925-2930.

506. Vento S, Cainelli F, Longhi MS. Reactivation of replication of hepatitis B and C viruses after immunosuppressive therapy: an unresolved issue. Lancet Oncol 2002;3:333-340.


507. Faggioli P, De Paschale M, Tocci A, Luoni M, Fava S, De Paoli A, et al. Acute hepatic toxicity during cyclic chemotherapy in non HodgkinŌĆÖs lymphoma. Haematologica 1997;82:38-42.

508. Nosotti L, DŌĆÖAndrea M, Pitidis A, Pimpinelli F, Dessanti ML, Pisani F, et al. Hepatitis C virus infection prevalence and liver dysfunction in a cohort of B-cell non-HodgkinŌĆÖs lymphoma patients treated with immunochemotherapy. Scand J Infect Dis 2012;44:70-73.


509. Takai S, Tsurumi H, Ando K, Kasahara S, Sawada M, Yamada T, et al. Prevalence of hepatitis B and C virus infection in haematological malignancies and liver injury following chemotherapy. Eur J Haematol 2005;74:158-165.


510. de Pree C, Giostra E, Galetto A, Perrin L, Zulian GB. Hepatitis C virus acute exacerbation during chemotherapy and radiotherapy for oesophageal carcinoma. Ann Oncol 1994;5:861-862.


511. Melisko ME, Fox R, Venook A. Reactivation of hepatitis C virus after chemotherapy for colon cancer. Clin Oncol (R Coll Radiol) 2004;16:204-205.


512. Fan FS, Tzeng CH, Hsiao KI, Hu ST, Liu WT, Chen PM. Withdrawal of immunosuppressive therapy in allogeneic bone marrow transplantation reactivates chronic viral hepatitis C. Bone Marrow Transplant 1991;8:417-420.

513. Kanamori H, Fukawa H, Maruta A, Harano H, Kodama F, Matsuzaki M, et al. Case report: fulminant hepatitis C viral infection after allogeneic bone marrow transplantation. Am J Med Sci 1992;303:109-111.


514. Vento S, Cainelli F, Mirandola F, Cosco L, Di Perri G, Solbiati M, et al. Fulminant hepatitis on withdrawal of chemotherapy in carriers of hepatitis C virus. Lancet 1996;347:92-93.


515. Nakamura Y, Motokura T, Fujita A, Yamashita T, Ogata E. Severe hepatitis related to chemotherapy in hepatitis B virus carriers with hematologic malignancies. Survey in Japan, 1987-1991. Cancer 1996;78:2210-2215.


516. Locasciulli A, Bruno B, Alessandrino EP, Meloni G, Arcese W, Bandini G, et al. Hepatitis reactivation and liver failure in haemopoietic stem cell transplants for hepatitis B virus (HBV)/hepatitis C virus (HCV) positive recipients: a retrospective study by the Italian group for blood and marrow transplantation. Bone Marrow Transplant 2003;31:295-300.


517. Hamaguchi M, Yamada H, Gondo H, Takemoto Y, Morishima Y, Kodera Y. Retrospective study on the impact of hepatitis B and hepatitis C virus infection on hematopoietic stem cell transplantation in Japan. Int J Hematol 2002;75:324-331.


518. Lee JM, Lee JM, Yoo HS, Jang UK, Kim DJ, Kim YB, et al. The prevalence of anti-HCV positivity in healthy Korean children. Korean J Hepatol 1996;2:160-165.
520. Pembrey L, Newell ML, Tovo PA. EPHN Collaborators. The management of HCV infected pregnant women and their children European paediatric HCV network. J Hepatol 2005;43:515-525.


521. Airoldi J, Berghella V. Hepatitis C and pregnancy. Obstet Gynecol Surv 2006;61:666-672.


522. Hepatitis C virus infection. American Academy of Pediatrics. Committee on Infectious Diseases. Pediatrics 1998;101(3 Pt 1):481-485.


523. Mast EE, Hwang LY, Seto DS, Nolte FS, Nainan OV, Wurtzel H, et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis 2005;192:1880-1889.


524. Palomba E, Manzini P, Fiammengo P, Maderni P, Saracco G, Tovo PA. Natural history of perinatal hepatitis C virus infection. Clin Infect Dis 1996;23:47-50.


525. Polywka S, Pembrey L, Tovo PA, Newell ML. Accuracy of HCV-RNA PCR tests for diagnosis or exclusion of vertically acquired HCV infection. J Med Virol 2006;78:305-310.


526. Guido M, Rugge M, Jara P, Hierro L, Giacchino R, Larrauri J, et al. Chronic hepatitis C in children: the pathological and clinical spectrum. Gastroenterology 1998;115:1525-1529.


527. Mack CL, Gonzalez-Peralta RP, Gupta N, Leung D, Narkewicz MR, Roberts EA, et al. NASPGHAN practice guidelines: diagnosis and management of hepatitis C infection in infants, children, and adolescents. J Pediatr Gastroenterol Nutr 2012;54:838-855.


528. El Sherbini A, Mostafa S, Ali E. Systematic review with meta-analysis: comparison between therapeutic regimens for paediatric chronic hepatitis C. Aliment Pharmacol Ther 2015;42:12-19.


529. Gonz├Īlez-Peralta RP, Kelly DA, Haber B, Molleston J, Murray KF, Jonas MM, et al. Interferon alfa-2b in combination with ribavirin for the treatment of chronic hepatitis C in children: efficacy, safety, and pharmacokinetics. Hepatology 2005;42:1010-1018.


530. Wirth S, Lang T, Gehring S, Gerner P. Recombinant alfa-interferon plus ribavirin therapy in children and adolescents with chronic hepatitis C. Hepatology 2002;36:1280-1284.


531. Wirth S, Pieper-Boustani H, Lang T, Ballauff A, Kullmer U, Gerner P, et al. Peginterferon alfa-2b plus ribavirin treatment in children and adolescents with chronic hepatitis C. Hepatology 2005;41:1013-1018.


532. Wirth S, Ribes-Koninckx C, Calzado MA, Bortolotti F, Zancan L, Jara P, et al. High sustained virologic response rates in children with chronic hepatitis C receiving PegIFN-a alfa-2b plus ribavirin. J Hepatol 2010;52:501-507.


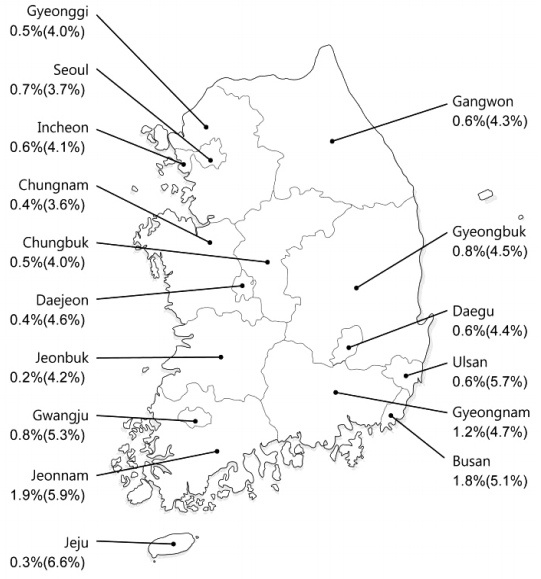




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



