Strategic application of radiotherapy for hepatocellular carcinoma
Article information
Abstract
With increasing clinical use, radiotherapy (RT) has been considered reliable and effective method for hepatocellular carcinoma (HCC) treatment, depending on extent of disease and patient characteristics. RT for HCC can improve therapeutic outcomes through excellent local control, downstaging, conversion from unresectable to resectable status, and treatments of unresectable HCCs with vessel invasion or multiple intrahepatic metastases. In addition, further development of modern RT technologies, including image-guided radiotherapy (IGRT), intensity-modulated radiotherapy (IMRT), and stereotactic body radiotherapy, has expanded the indication of RT. An essential feature of IGRT is that it allows image guidance therapy through in-room images obtained during radiation delivery. Compared with 3D-conformal RT, distinctions of IMRT are inverse treatment planning process and use of a large number of treatment fields or subfields, which provide high precision and exquisitely conformal dose distribution. These modern RT techniques allow more precise treatment by reducing inter- and intra-fractional errors resulting from daily changes and irradiated dose at surrounding normal tissues. More recently, particle therapy has been actively investigated to improve effectiveness of RT. This review discusses modern RT strategies for HCC, as well as optimal selection of RT in multimodal approach for HCC.
INTRODUCTION
Traditionally, the role of radiotherapy (RT) for hepatocellular carcinoma (HCC) has been limited due to the relatively low liver tolerance to radiation, although many are not candidates for curative treatment or not adequately treated with transarterial chemoembolization (TACE), radiofrequency ablation (RFA), or sorafenib. Wellknown Barcelona Clinic Liver Cancer (BCLC) guidelines for HCC did not recommend RT as a primary treatment option for all stages of HCC. In clinical guidelines of the European Association for the Study of the Liver and European Organization for Research and Treatment of Cancer (EASL-EORTC), use of external beam radiotherapy (EBRT) was also limited due to the possibility of radiation-induced liver disease (RILD), and reasons for the benefits of three-dimensional conformal radiotherapy (3D-CRT) have only been shown in uncontrolled studies.
However, with increasing clinical use, RT has been considered reliable and effective for HCC treatment, depending on each stage. In addition, further development of modern RT technologies, including intensity-modulated radiotherapy (IMRT), image-guided radiotherapy (IGRT), and stereotactic body radiotherapy (SBRT), have expanded the indication of RT. In comparison to 3D-CRT, distinctions of IMRT include inverse treatment planning process and use of a large number of treatment fields or subfields, which provide high precision and exquisitely conformal dose distribution. Furthermore, the addition of multileaf collimator (MLC) enables non-uniform intensity profiles in various ways.
The role of RT is documented in several clinical guidelines. According to the most recent National Comprehensive Cancer Network (NCCN) guideline [1], EBRT is suggested as a locoregional treatment option for patients with unresectable HCC who are ineligible for transplantation, inoperable due to comorbidities, or those who have a local disease with/without minimal extrahepatic disease, with evidence level of 2B. Locoregional therapy, including EBRT, is also suggested as a treatment option for patients who have operable tumors, although resection is the preferred treatment. The guideline also mentions feasibility of modern RT and indications for SBRT. Korean Liver Cancer Study Group (KLCSG) has published the first comprehensive guidelines in 2003 with details regarding RT indication, and these guidelines have been updated recently in 2014 [2]. The KLCSG guideline provides multiple treatment suggestions for each stage based on the modified Union for International Cancer Control (mUICC) staging system, and lists the best options and alternatives. Based on recent perspectives, KLCSG is currently revising its 2014 guideline and discussing ways to further refine and expand the role of RT.
In this review, we describe the technical aspects of modern RT techniques for HCC along with their clinical applications in HCC, mainly according to the mUICC staging system. Furthermore, we introduce recent trends of RT, such as particle therapy.
MODERN RADIOTHERAPY TECHNIQUES
Intensity-modulated radiotherapy (IMRT)
Development of RT mainly focuses on improving two factors: accuracy and precision. Accuracy of RT implies that radiation can be delivered to the correct location each time, despite various uncertainties between the time of radiation treatment planning and the time of actual treatment. Precision of RT means the ability to control distribution of radiation to make sure that prescribed dose is administered exactly to tumor site, and that radiation is not delivered to surrounding normal tissues.
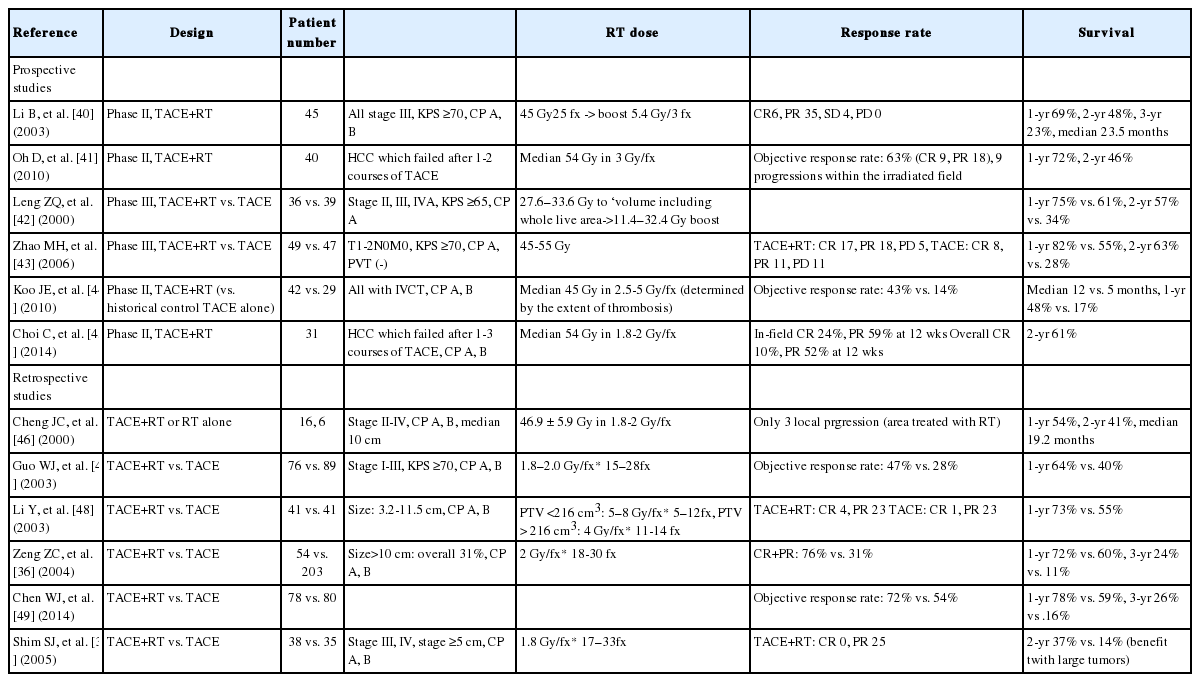
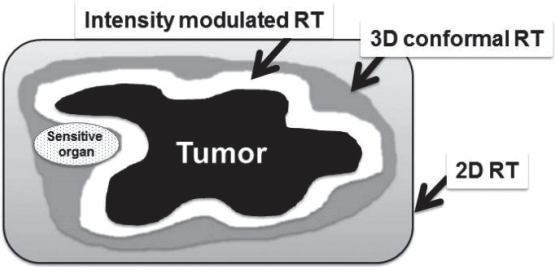
In the past, it was possible to perform treatment using two-dimensional fluoroscopic imaging plan and estimate radiation distribution in only one direction (“2D-RT”). Based on the development of computer engineering and radiology, computerized tomography (CT) images were used in planning simulation, while considering the three-dimensional positional relationship between tumor and surrounding normal tissues, in addition to more conformal treatments (“3D-CRT”). IMRT is the most advanced 3D-conformal treatment technique. It modulates the intensity of radiation delivered in each treatment direction, and selectively delivers the desired dose of radiation only to the tumor site while minimizing the amount of radiation entering normal tissue around tumor (Fig. 1). The core of this treatment technique is the so-called “inverse treatment planning,” in which the physician sets the ideal dose of targets and normal organs, and relative importance for tumor and surrounding organs in advance. Then, the machine computes and determines numbers and angles of beams. In the past, physicians performed target contouring and determined numbers and angles of beams, and then the machine performed RT (“Forward planning”) (Fig. 2).
Comparison of treatment volumes by various radiotherapy RT technology involving 2D radiotherapy, 3D conformal radiotherapy (3D-CRT), and intensity modulated radiotherapy (IMRT). IMRT is the most advanced 3D-conformal treatment technology. It modulates the intensity of radiation delivered in each treatment direction, and selectively delivers the desired dose of radiation only to the tumor site, while minimizing the amount of radiation entering sensitive normal organs around the tumor with high precision.
Concept of inverse planning of intensity modulated radiotherapy (IMRT). In the past, physicians performed target contouring and determined numbers and angles of the beams, and then the machine performed radiotherapy (“forward planning”). The core of IMRT technique is the socalled “inverse planning,” in which the physician sets ideal dose of targets and normal organs, and relative importance for the tumor and surrounding organs in advance. Then, the machine computes and determines numbers and angles of the beams.
Control of respiratory motion during radiation delivery
Another critical issue in RT for HCC is the control of respiratory motion, as the liver moves in a considerable range during respiration. There are several ways to treat a moving tumor to ensure the tumor receives intended dose while reducing the dose to surrounding normal tissue. The strategies can be classified into motion-encompassing, breath-hold, forced shallow breathing with abdominal compression, respiratory gating, and real-time tumor tracking.
Motion-encompassing method refers to the covering of all possible positions of moving tumor through the entire breathing cycle using 4D-CT images. Subsequently, a large volume of normal tissue may be irradiated. Breath-hold method refers to letting the patient hold his or her breaths for a few seconds under deep inspiration, and then deliver radiation only when the liver is in a certain position. Forced shallow breathing is using a particular external device, such as an abdominal compressor, to allow the patient to breathe shallow during radiation therapy. Although breath-hold and forced shallow breathing might result in patient discomfort or inconvenience during treatment, it can reduce respiratory motion for liver tumors and enhance accuracy.
Currently, respiratory gating and real-time tumor tracking are considered the most advanced techniques. Respiratory gating method involves turning on the radiation beam only during a specific respiratory cycle, after accurately grasping the position of tumor according to the patient’s respiratory cycle in advance, using 4D-CT images.
Real-time tracking method refers to tracking the movement of tumor along respiratory cycle using the surrogate on abdominal surface or internal fiducial marker, and then delivering radiation by following tumor inside the body. No respiratory control and abdominal compression are needed. This gating method using an external breathing signal is natural, noninvasive, and radiation-free; however, potential error might be that the signal does not accurately correlate with internal target position. On the other hand, the use of internal fiducial markers is an invasive method; however, it can improve the accuracy of treatment. Currently, one of the machines that are capable of tumor tracking is the CyberKnife system. Clinical feasibility of CyberKnife system has been demonstrated in several studies, especially SBRT papers. CyberKnife SBRT has been proven to be a safe and effective noninvasive treatment for HCC [3-7]. CyberKnife system consists of a pair of fluoroscopes in the ceiling coupled to a small X-band linear accelerator mounted on robotic arm, which can move according to the movement of inserted fiducial markers. Patients are encouraged to undergo implantation of three or more fiducial markers in the liver parenchyma around the perimeter of tumor before treatment, which would serve as radiographic landmarks for respiratory synchrony system and image guidance technique.
Image-guided radiotherapy (IGRT)
IGRT is defined as RT that utilizes imaging to maximize accuracy throughout the entire treatment process, including precise targeting and normal tissue representation, radiation delivery, and adaptive plans for anatomic and biological changes over time for the patient. Of these, accurate target delineation, target relocalization to allow proper patient repositioning, and respiratory motion management have been the most challenging for patients with HCC, mainly due to the uncertain movement of the liver.
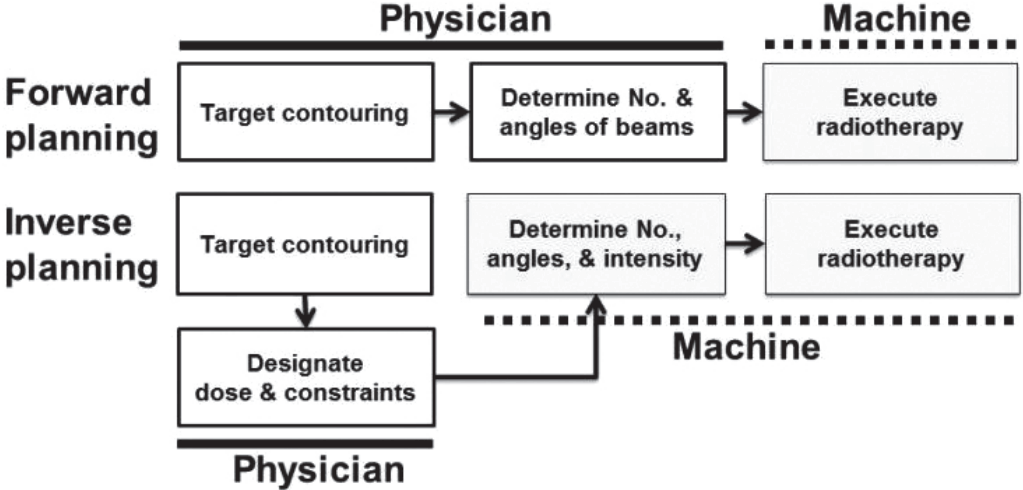
An essential feature of IGRT is that it allows image guidance therapy through in-room images obtained during radiation delivery (Fig. 3). This leads to more precise treatment by reducing inter- and intra-fractional errors that result from daily changes in the liver to bones, breathing motion, and variation in shape and position of neighboring organs. Physicians can reduce target margins and spare additional normal tissue dose using 2D or 3D image guidance techniques (helical megavoltage CT, kilovoltage cone-beam CT (CBCT), and MV CBCT), compared to in-room laser beams and skin marks (Fig. 3). Degree of error is evaluated by comparing reference images to in-room images. If the error is beyond allowable range and physicians see a need for adaptive planning, they should retake planning CT and restart treatment after new RT planning (Fig. 4).
Illustration of image-guided radiotherapy (IGRT) with daily setup using three-dimensional volumetric imaging modality. Matching between “reference images” using three-dimensional reconstruction of planning computed tomography images and in-room cone-beam computed tomography images are performed on each day of treatment.
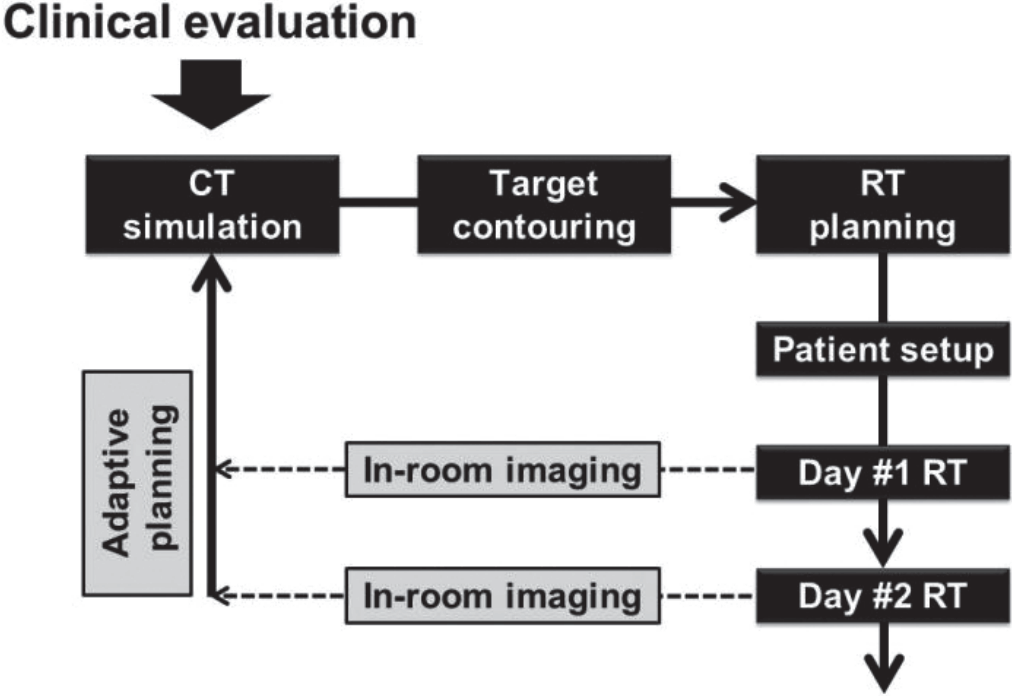
Workflow of image-guided radiotherapy (IGRT). An essential feature of IGRT is that it allows image guidance therapy through in-room images obtained during radiation delivery. Physicians can reduce target margins and spare additional normal tissue dose using 2D or 3D image guidance techniques (helical megavoltage CT (MVCT), kilovoltage cone-beam CT (CBCT), and MV CBCT), compared to in-room laser beams and skin marks. Degree of error is evaluated by comparing reference images with in-room images at every treatment time. If the error is beyond the allowable range and physicians sees a need for adaptive planning, they should retake a planning CT and restart treatment after new RT planning.
RADIOTHERAPY FOR EARLY STAGE (mUICC STAGE I/II) HCC
Curative therapies can improve survival in early-stage HCC patients. Resection, percutaneous ethanol injection (PEI), and RFA have been considered valid local ablative treatments. However, when the effect is limited for reasons of primary tumor location (e.g., near the liver dome, or major vessels), RT may be considered as an alternative. Also, RT may be a feasible alternative for those who are inoperable or refuse surgery.
SBRT for early stage HCC
Local high-dose RT, including SBRT, can be an appropriate alternative definitive or salvage treatment. In several prospective [8-17] (Table 1) or retrospective [3,4,18-29] (Table 2) papers, SBRT has been reported as a very effective, safe, and noninvasive treatment modality in primarily diagnosed or recurrent cases when size and position are acceptable. Honda Y, et al. demonstrated that SBRT combined with TACE is a safe and effective modality for locoregional treatment of small solitary primary HCC, with a 96% of high complete response (CR) rate [22]. Even for huge HCC ≥10 cm, SBRT could be a safe and effective treatment option. Jang WI, et al. suggested that there was a positive linear relationship between SBRT dose and 2-yr local control, overall survival (OS) rates, which were highest at >54 Gy [24]. Sometimes, SBRT was also used for portal vein tumor thrombosis (PVTT) and/or inferior vena cava tumor thrombosis [23,27], BCLC stage C HCC [27], or huge HCC ≥10 cm [28,29], with acceptable response rates and survival outcomes. Wahl DR, et al. compared outcomes of patients with inoperable, nonmetastatic HCCs who underwent SBRT or RFA [30]. For tumors <2 cm, there was no significant difference between RFA and SBRT in freedom from local progression (FFLP) (HR, 2.50; 95% CI, 0.72 to 8.67; P=0.15); however, for tumors ≥2 cm, RFA was associated with significantly worse FFLP (HR, 3.35; 95% CI, 1.17 to 9.62, P =0.025).
Radiotherapy as bridging treatment before liver transplantation
O’Connor JK, et al. reported that SBRT could be used as effective bridging treatment before liver transplantation [31]. In a recent study from Toronto General Hospital [32], 379 patients who underwent liver transplantation after SBRT (n=36), TACE (n=99), or RFA (n=244) were compared, to evaluate the safety and efficacy of bridging treatments. Drop-out rate and postoperative complication rates were similar between groups. The 3-year survival rate from time of transplant was 75% in SBRT group, 75% in TACE group, and 81% in RFA group, which were not significantly different. This showed that SBRT might be as effective and safe as TACE or RFA, when used to maintain candidacy of patients with HCC who are on the wait-list for a transplant for the first time.
RADIOTHERAPY FOR INTERMEDIATE STAGE (mUICC STAGE II/III) HCC
In general, intermediate stage HCC is considered primarily for TACE. However, the effects of TACE may be limited if there is vascular shunting, recanalization around the tumor capsule, or development of multiple feeding vessels. TACE is contraindicated in patients with PVTT or inferior vena cava invasion, as it has potential risk of ischemic liver damage [33]. In addition, complete or massive necrosis is seldom observed following TACE, when the tumor is >5 cm [34,35]. In HCCs that either showed incomplete response after TACE or were unsuitable for TACE, RT can be useful as a complementary modality [36-39].
Radiotherapy for unresectable HCC after incomplete TACE
In many prospective [40-45] or retrospective [36,39,46-49] papers, large unresectable HCCs were well treated with TACE followed by EBRT, and objective response rates (complete and partial responses) were achieved in about 63-76% of cases with 72-82% of 1-year survival rate, which is significantly higher compared to patients without EBRT (Table 3). A recent prospective phase II multicenter study [45] investigated the efficacy and toxicity of RT following incomplete TACE in unresectable HCCs. Here, patients with unresectable HCC who had viable tumor after TACE of no more than three courses were eligible, and median 54 Gy of 3D-CRT was delivered. Best objective infield response rate was achieved in 84% of patients, with 23% of CR rates and 61% of partial response (PR) rates within 12 weeks post-RT. The 2-year in-field progression-free survival (PFS), overall PFS, and OS rates were 45%, 29%, and 61%, respectively. These findings demonstrate that early application of 3D-CRT can be a promising option in multimodal approaches for patients with incomplete necrosis after TACE. Meng MB, et al. performed meta-analysis from five randomized controlled trials and 12 nonrandomized controlled clinical trials, which compared TACE+RT group and TACE alone group [50]. TACE+RT significantly improved survival rates and CR rates (OR, 2.58; 95% CI 1.64–4.06; P=0.0001). Rates of adverse events were not significantly different, except for elevation of total bilirubin level. Other meta-analyses [51,52] also presented similarly favorable outcomes with TACE+RT compared to using TACE alone.
RADIOTHERAPY FOR ADVANCED STAGE (mUICC STAGE III/IV) HCC
Radiotherapy for unresectable HCC with PVTT
For patients who are unsuitable for TACE due to PVTT, value of RT has been especially noticeable in several prospective [44,53-57] and retrospective studies [38,58-67]. About 31% to 83% of objective response rates and median OS of 7 months to 34 months in responders have been reported [37,59,61,67,68] (Table 4). According to Korea’s long-term follow-up data [59], authors reported clinical outcomes of patients who underwent RT for HCC with PVTT. With radiation volume including PVTT (±whole HCC), median survival time was 10.6 months, and 1-year survival rate was 43%. Furthermore, 3.6% of patients achieved CR and 24.3% of patients achieved PR, with 10% of grade 3-4 hepatic toxicity and 4% of gastroduodenal complications. TACE plus RT achieved significant survival advantage in HCC with PVTT than using TACE alone, according to propensity score matching [69]. Median survival time was 10.9 versus 4.1 months (P<0.001) in all patients, 12.5 versus 4.4 months (P =0.002) in patients with PVTT involving the right/left portal vein, and 8.9 versus 4.0 months (P<0.001) in patients with PVTT involving the main portal vein trunk. Based on another paper [62], TACE+RT showed longer OS in selected patients with locally advanced HCC in BCLC stage C who had macrovascular invasion in 96% of cases and received RT to vascular invasions, compared to sorafenib event after propensity score matching. Tang QH, et al. [61] also compared the outcomes of surgical resection plus 3D-CRT to tumor and PVTT with resection alone. There was a median survival advantage of 2.3 months (P =0.03), and 1-year survival rate was 52%. Stereotactic ablative radiotherapy (SABR) (median 40 Gy in 4-5 fractions) using CyberKnife was also suggested as a feasible treatment option [70]. In a multicenter retrospective study in Korea [67], outcomes of 985 patients who received RT for PVTT (±whole HCC) were analyzed, and response rate of PVTT was reported as 52%. Responders had significantly more prolonged survival (15 vs. 10 months) and equivalent RT dose >45 Gy when given in combination with other treatments, and provided significantly better PVTT control and OS.

Clinical outcomes of RT-combining treatments for hepatocellular carcinoma with portal vein thrombosis
Recently, meta-analysis results of eight clinical studies regarding this subject were published in China [71]. TACE+RT significantly improved objective response rate (OR, 4.22; 95% CI, 3.07–5.80; P<0.001) of PVTT and OS (HR, 0.69; 95% CI, 0.57–0.83; P =0.001), compared to TACE alone, although incidence of grade 3 or 4 leukopenia and thrombocytopenia was significantly higher.
Radiotherapy for huge HCC
RT can be very challenging to perform in some cases of very large unresectable HCCs. Although TACE has been frequently used in treatment of unresectable HCC, its limitation has also been well known, especially in large tumors, particularly regarding complicated blood supply and high incidence of residual viable tumor even after repeated treatment. Although surgical resection could also be tried in some cases, its indication is very limited. Shim SJ, et al. showed that TACE+RT offered more significant benefit than TACE alone when tumor size was larger [37]. According to the specific tumor size, 2-year survival rates in TACE+RT and TACE groups were 63% vs. 42% in 5–7 cm (P=0.22), 50% vs. 0% in 8–10 cm (P=0.03), and 17% vs. 0% in larger than 10 cm (P=0.002), respectively. In addition, Kim KH, et al. tried to find the most optimal treatment for huge HCCs ≥10 cm in patients who received various treatments at the same single institution [72]. Median OS was longer in TACE+RT group (15.3 months) and concurrent chemoradiotherapy (CCRT) group (12.8 months), compared to TACE alone group (7.5 months) and hepatic arterial infusion chemotherapy (HAIC) alone group (8.2 months); this indicated that huge unresectable HCCs treated with RT, either as CCRT or in combination with TACE, showed excellent intrahepatic control and prolonged survival.
RT for HCC with multiple intrahepatic metastases
The role of local RT is more uncertain for HCC with multiple intrahepatic metastases. In one retrospective study [73], local RT to the main tumor was most beneficial with well-controlled intrahepatic tumors out of RT field. Patients with viable intrahepatic tumors out of RT field showed worse survival. Survival was similar when all lesions were covered by RT field, or lesions out of RT field were controlled by TACE. In a more recent study [57], TACE for intrahepatic metastases and localized CCRT were given to HCCs with portal vein invasion and intrahepatic metastasis. Objective response rate was 32.1%, and median PFS and OS were 4.5 months and 9.8 months, respectively. Incidence of grade 3–4 toxicity was low, manageable, and predictable, although two patients dropped out due to grade 3 nausea and vomiting.
Concurrent chemoradiotherapy for advanced stage HCC
Several studies have indicated that hepatic arterial infusion concurrent chemoradiation (HAICCRT) may be a feasible and effective alternative for unresectable liver-confined HCC with vascular invasion. The purpose of using HAIC in CCRT was to enhance local radio-therapeutic effect and to reduce intrahepatic HCC spread.
One pilot clinical study reported that CCRT improved response rates and survival for locally advanced HCC with portal vein invasion [54]. One month after localized CCRT, objective response was observed in 45% of patients, and 3-year survival rate was 24% which was significantly better compared to non-responders. More recently, the same institution showed treatment outcomes of 30 HCCs with portal vein invasion and intrahepatic metastasis [57]. After TACE for intrahepatic metastasis, localized CCRT (45 Gy in 25 fractions) was used to treat the main HCC with PVT. Objective response rate was about 30% and median OS was 9.8 months, without any severe adverse events. Review of nationwide multi-center HCC cohort (stage III-IV, CP-A) showed that patients who underwent definitive CCRT as initial treatment showed significantly better OS (median 11.4 versus 6.6 months, P =0.02) than matched patients who did not receive CCRT [74]. CCRT followed by HAIC in locally advanced HCC could even increase resectability by down-staging tumors and increasing functional residual liver volume, and 5-year OS was significantly increased to 50% (versus 10%) [75,76]. In addition, EBRT can significantly relieve symptoms that are caused by locally advanced HCC or metastatic tumors, and even a prolongation of survival period can be expected [77,78].
DOSE CONSTRAINTS
Development of improved treatment planning and dose delivery methods, such as 3D-CRT and IMRT, provided a mechanism not only to target hepatic lesions while sparing uninvolved hepatic parenchyma but also to precisely measure radiation dose delivered to both tumor volume and surrounding normal tissue. Despite technical advances, RILD still remains to be a side effect that presents significant concerns when planning RT for HCC.
RILD is a clinical syndrome of anicteric hepatomegaly, ascites, and elevated liver enzymes (particularly serum alkaline phosphatase) that occurs typically 2 weeks to 4 months after completion of hepatic irradiation. Tolerance of the whole liver to radiation is low, and RILD is seen in 5–10% of patients treated with 30–35 Gy to the whole liver, based on several Radiation Therapy Oncology Group (RTOG) studies in the 1970’s and 1980’s. For this reason, radiation has traditionally played a limited role in the treatment of liver tumors. However, treatment of liver parts using higher radiation doses is possible without adverse effects, as long as an adequate volume of normal liver is saved.
While the Emami report established baseline liver tolerance doses based on literature reports of toxicity [79], later studies provided more detailed assessment and guidelines regarding the risk of hepatotoxicity for given RT doses. At the University of Michigan [80], dose-volume tolerance for RILD using the Lyman-Kutcher-Burman (LKB) normal tissue complication probability (NTCP) model was described. They demonstrated that the liver exhibits a large volume effect for RILD, suggesting that the mean liver dose may be useful in ranking radiation plans. No cases of RILD were observed when the mean liver dose was <31 Gy. Furthermore, multivariate analysis demonstrated that, in addition to NTCP and the mean liver dose, a primary hepatobiliary cancer diagnosis (vs. liver metastases), bromodeoxyuridine hepatic artery chemotherapy (vs. fluorodeoxyuridine chemotherapy), and male gender were associated with RILD. In a preclinical study [81] that treated rat cirrhosis model with mildly impaired liver function, combined treatment of partial RT plus 5-FU resulted in a significantly high incidence of lethal liver injury. According to Dawson LA’s review paper [82], if the effective liver volume irradiated is less than 25%, very high RT doses (>100 Gy) may be delivered with little risk of liver toxicity, as long as the liver function is proper. In addition, RT tolerance of the liver is reduced in patients with primary liver cancer versus metastases. The mean liver doses associated with 5% risk of classic RILD for primary and metastatic liver cancer are 28 Gy and 32 Gy, respectively, in 2 Gy per fraction.
According to recent papers [83,84], there are several dose-volumetric parameters related to the risk of RILD, which are also included in the 2014 KLCSG guidelines. Tumor volume must be limited to ≤70% of the total liver volume, and liver volume receiving ≥30 Gy must be constrained to ≤60% of total liver volume on dose-volume histograms for 3D-RT planning. For SBRT, normal liver volume receiving a total dose of <15 Gy must be ≥700 mL, or the mean normal liver dose (liver minus gross tumor volume) must be limited to <28 Gy in 2-Gy fractions. Although additional studies for liver tolerance to radiation are still needed, several institutions have similarly applied dose–volume histogram parameter-based RT guidelines.
CHARGED PARTICLE THERAPY
Charged particle therapy (CPT), such as proton and carbon ion therapy, is a form of radiotherapy with superior depth dose distribution compared to photon radiotherapy. This superiority in depth dose distribution is attained by the energy-dependent specific range of charged particles within the tissues and the Bragg peak, which is the sharp peak of energy deposit just before stopping the particle. Consequently, tumors can be treated more efficiently with less toxicity by charged particles than by photons on theoretical grounds, even in the cirrhotic liver with limited hepatic functional reserve. Some retrospective and prospective studies have reported encouraging outcomes with proton or carbon beam therapy in patients with HCC. Local control rates were 88%-98% at 2-5 years, with very low incidence of severe toxicity.
Proton beam therapy
At the University of Tsukuba, long-term results of proton beam therapy (PBT) for HCCs were reported [85-88]. A total dose of 77.0 gray-equivalent (GyE) in 35 fractions was administered for tumors located within 2 cm of a digestive organ, 72.6 GyE in 22 fractions was administered for tumors located within 2 cm of the porta hepatis, and 66.0 GyE in 10 fractions was administered for peripheral tumors located more than 2 cm from both GI tract and porta hepatis. The 5-year survival was 23.5%, and local control rate was 86.9%. For a patient subgroup having BCLC stage 0/A HCCs, 5-year local control, PFS, and OS rates were much higher (94%, 28%, and 69%). Otherwise, there were very few acute reactions to treatment, in addition to a few grade 2 or more severe sequelae. So far, the largest prospective study of PBT for HCC was reported by the Loma Linda University Medical Center [89,90]. After 63 GyE in 15 fractions of PBT, 20% had experienced local treatment failure, and median PFS was 36 months. No acute toxicity that required treatment interruption was found; however, five cases of grade 2 gastrointestinal bleeding or ulceration near the irradiated area were observed in patients who were treated in earlier period. In another phase II study of MGH [91,92], 67.5 GyE and 58.05 GyE in 15 fractions for peripheral and central tumors were delivered, and 94% of favorable 2-year local control rate was reported. No local recurrence was reported with ≥60 GyE, and median PFS and OS were 13.9 months and 49.9 months, respectively. In other prospective studies [93-95], PBT showed about 95% of 2-year local control rates and 63-66% of 2-yr OS rates (Table 5). In a recent interim analysis of prospective randomized clinical trial comparing PBT and TACE [96], 2-year local control and PFS rates were higher with PBT (88% vs. 45%, 48% vs. 31%), although they were not significantly different. The total number of hospitalization days was significantly shorter in patients with PBT (24 vs. 166 days, P<0.001). Pathologic complete response was achieved in 10% of TACE group and 25% of PBT group, who underwent liver transplantation after treatment. There was a trend toward improved 2-year local control (88% vs. 45%, P=0.06) and PFS (48% vs. 31%, P=0.06) favoring PBT.
Excellent local tumor control was reported, even in tumors with portal vein invasion (Table 5) [97-99]. Sugahara S, et al. [98] demonstrated that 2-year PFS and OS rates were 91% and 33-57% after PBT for HCC with PVTT, without treatment-related severe complications. Kim, et al. [93] also treated HCC with PVT, and local recurrence was noted in only 12% of patients during follow-up period, without local recurrence or severe gastrointestinal toxicity. In addition, proper local control rates can be expected for HCC patients with large tumors (87% at 2 years for tumors >10 cm) or portal tumors (86% at 3 years), as well as for elderly patients (100% at 3 years for patients aged ≥80 years) [100-103]. Even for recurrent tumors after PBT, repeated PBT can be safely delivered with an excellent local control rate of 87.8% at 5 years [104].
With concerns about gastrointestinal toxicities and hepatic insufficiencies, Kawashima M, et al. [105] analyzed dose-volume histogram of 60 patients with HCC who were treated by PBT, and reported ICG R15 and V30 as useful predictors. According to the location of tumor, a risk-adapted simultaneous integrated boost technique could be utilized to avoid gastrointestinal toxicities. When PTV overlaps the planning organ at risk volume (PRV) of gastrointestinal tract, 50–60 GyE in 10 fractions was prescribed to PTV minus the overlapping volumes, whereas the dose to overlapping volumes was restricted to 30 GyE in 10 fractions [106]. Mizumoto M, et al. [86] also suggested risk-adapted selection scheme dose fractionation schedules. They adopted a small fraction dose schedule of 77 GyE in 35 fractions with gastrointestinal tract avoidance as far as possible, after 40–50 GyE for tumors proximal to the alimentary tract with reduced risk of gastrointestinal toxicities.
Carbon ion beam therapy
Although it is expected that the biological benefits of high relative biological effectiveness (RBE) and high linear energy transfer (LET) of carbon ion beam would offer better treatment efficacy than photon or PBT, clinical experience for carbon ion beam is not yet extensive.
The first clinical result of carbon ion therapy for HCC was published by the NIRS in 2004 [107]. In 24 patients who were enrolled in this study and received 49.5 GyE-79.5 GyE in 15 fractions, 3-year local control and OS rates were 81% and 50%, respectively. The OS rates of patients who had not received previous treatment for HCC were significantly higher than those who did. In particular, there was no local recurrence in patients receiving more than 72 GyE, and severe adverse events were not reported at any dose level. To date, NIRS researchers have conducted several other clinical trials for HCC with short-course irradiation regimens. A four-fraction regimen, with a total dose of 52.8 GyE, also yielded a high local control rate of 94% at 3 years [108]. Although local control rates for porta hepatis lesions were slightly worse than those for non-porta hepatis lesions, the difference was not statistically significant (88 vs. 96%, P =0.306) [109]. Two-fraction regimens are now being adopted with minor adverse events only [110], and follow-up clinical data is not yet available. Other hypofractionated regimens with dose escalation have been also studied, and mature data will be reported in the near future.
Komatsu S, et al. compared the clinical outcomes of PBT (278 tumors) to carbon ion therapy (108 tumors) in patients who were treated at the same institution [111]. The 5-year local control rates for PBT and carbon ion were 90% and 93%, respectively. For tumors <5cm, local control rates were similar between two modalities (96% vs. 95%); however, the rates were slightly lower with PBT than carbon ion therapy for larger tumors (84% vs. 91% for tumors of 5-10cm; 43% vs. 80% for tumors >10cm). In meta-analysis of 70 non-comparative observational studies (73 cohorts; 53 with photon therapy, and 20 with CPT) [112], the median radiation dose was higher in CPT cohorts compared to SBRT and conventional RT cohorts, while the median rate of patients with child-pugh A class was higher in CRT cohorts than in other cohorts. Additionally, median tumor size, rate of male, rate of patients with ECOG PS 0–1, or median HCC patients with tumor vascular thrombosis did not significantly differ between groups. The OS, PFS, and local control rates were significantly higher for CPT than those for conventional RT, while they were similar to SBRT in patients with HCC. Furthermore, high-grade acute and late toxicities were lower for CPT compared to conventional RT or SBRT.
So far, CPT generally showed better local control and survival rates than photon-based RT series, although a direct comparison is difficult due to differences in patient characteristics. Although facilities for CPT have been limited to this day, the use of CPT is anticipated to increase in the near future.
Notes
Author contribution
SH Choi and J Seong contributed to this paper with conception and design of the study, literature review and analysis, drafting and critical revision and editing, and final approval of the final version.
Notes
Conflicts of Interest: The authors have no conflicts to disclose.
Abbreviations
BCLC
Barcelona Clinic Liver Cancer
CPT
Charged particle therapy
CR
complete response
CBCT
cone-beam CT
EASL-EORTC
European Association for the Study of the Liver and European Organization for Research and Treatment of Cancer
EBRT
external beam radiotherapy
FFLP
freedom from local progression
Gy
Gray
GyE
Gray-equivalent
HCC
hepatocellular carcinoma
IGRT
Imageguided radiotherapy
IMRT
Intensity-modulated radiotherapy
LET
linear energy transfer
mUICC
modified Union for International Cancer Control
MLC
multileaf collimator
NCCN
National Comprehensive Cancer Network
OS
overall survival
PR
partial response
PVTT
portal vein tumor thrombosis
PFS
progression-free survival
PBT
proton beam therapy
RILD
radiation-induced liver disease
RFA
radiofrequency ablation
RT
radiotherapy
SBRT
stereotactic body radiotherapy
3D-CRT
three-dimensional conformal radiotherapy
TACE
transarterial chemoembolization