| Clin Mol Hepatol > Volume 24(2); 2018 > Article |
|
ABSTRACT
Figure 1.
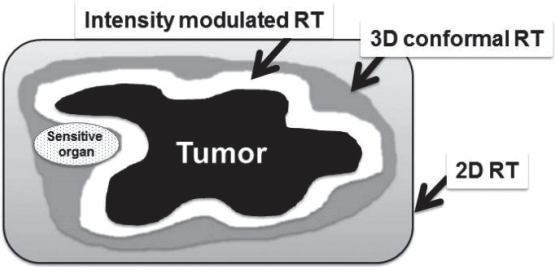
Figure 2.
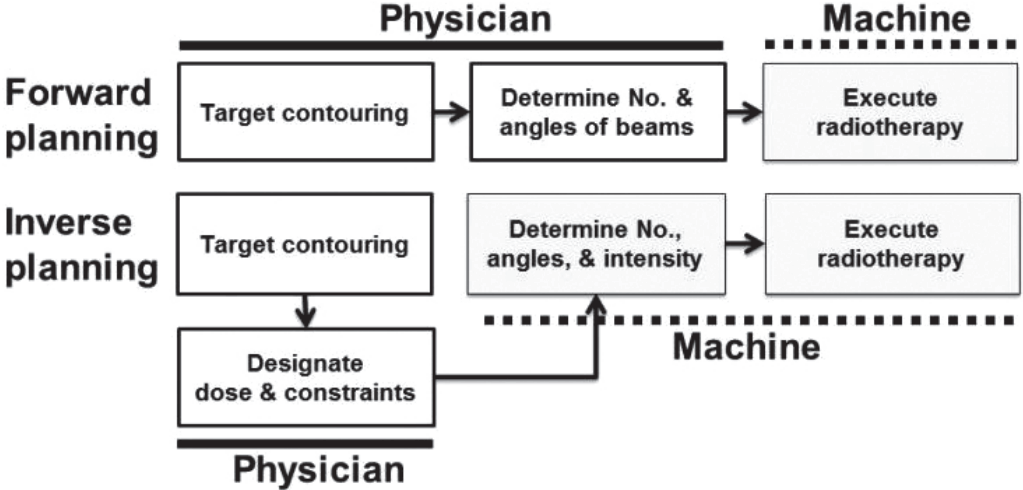
Figure 3.
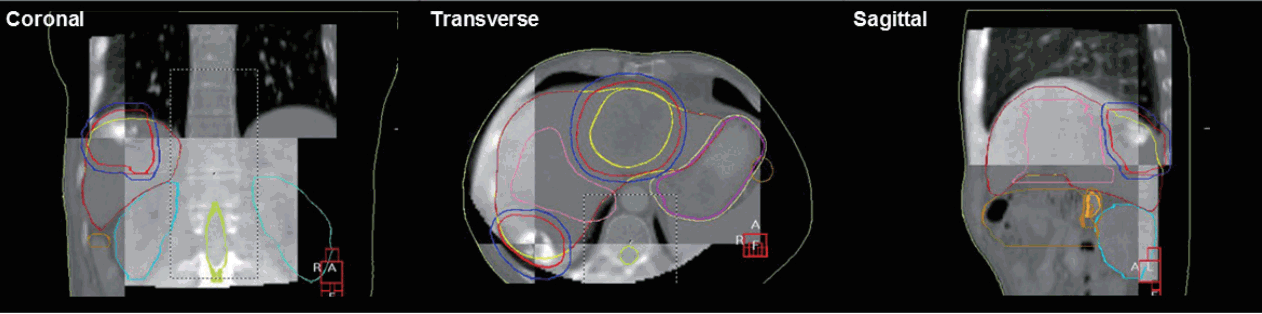
Figure 4.
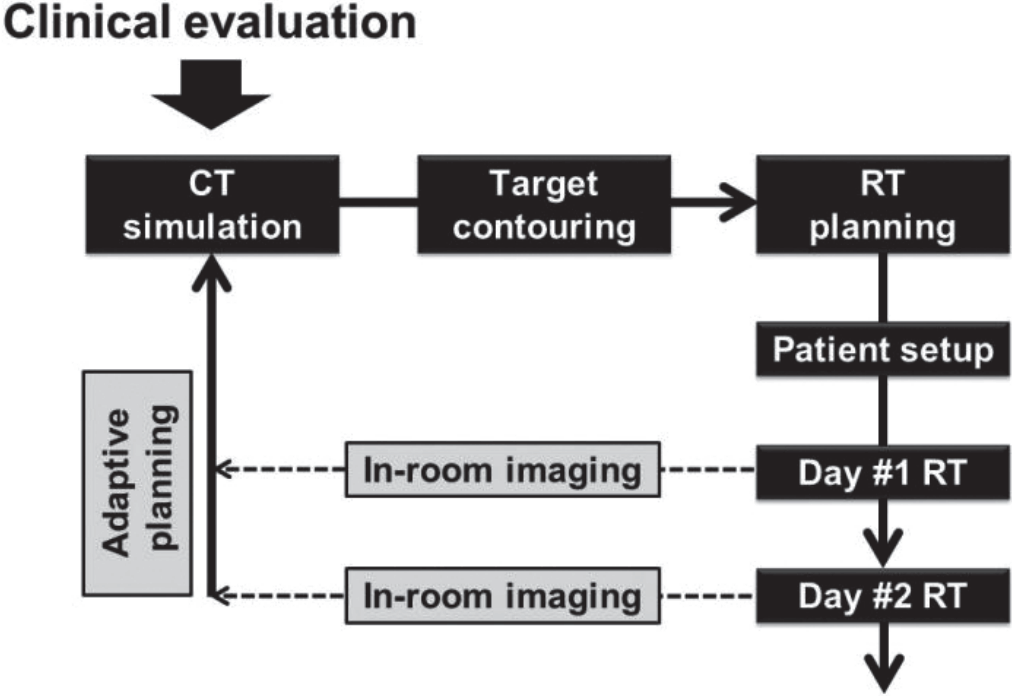
Table 1.
| Reference | Institutions | Design | RT Aim | Patient number | Indication | Median size (range), cm | Dose | Median f/u (range), mo | Local control | Overall survival |
|---|---|---|---|---|---|---|---|---|---|---|
| Herfarth KK, et al. [8] (2001) | Germany, Heidelberg Univ. | Phase I/II | Definitive/Salvage | HCC/CCC (4/54) | Unresectable liver tumor <3 tumors, <6 cm | Dose escalation 14-26 Gy/1 fx | 6 (1-26) | 81% (18 mo) | ||
| Tse RV, et al. [9] (2008) | Canada, Princess Margaret Hospital | Phase I | Definitive/Salvage | HCC/CCC (31/10) | CP-A, unresectable, previous Tx allowed | Median 36 (24-54) Gy/6 fx | 18 (11-39) | 65% (1 yr) | 48% (1 yr) | |
| Wulf J, et al. [10] (2006) | Switzerland | Prospective | Definitive | HCC+CCC/mets (5/51) | Unavailable for other Tx | Low dose : 30 Gy/3 fx or 28 Gy/4 fx High dose : 36-38 Gy/3 fx or 26 Gy/1 fx | HCC+CCC : 15 (2-48) | HCC+CCC : 83% | HCC+CCC : 76% (1 yr), 61% (2 yr) | |
| Goodman KA, et al. [11] (2010) | MSKCC | Phase I dose escalation | Definitive/Salvage | HCC/mets (2/24) | CP-A, unresectable, tumors < 5 | Dose escalation 18-30 Gy/1 fx | 17 (2-55) | 77% (1 yr) | 50% (2 yr) | |
| Cárdenes HR, et al. [12] (2010) | United States, Indiana Univ. | Phase I dose escalation | Definitive | All HCC (17) | CP-A, CP-B, 1-3 lesions, ≤ 6cm, PVT allowed, | CP-A : 36-48 Gy/3 fx CP-B : 40 Gy/5 fx | 24 (10-42) | 100% | 75% (1 yr) 60% (2 yr) | |
| Andolino DL, et al. [13] (2011) | United States, Indiana Univ. | Phase II | Definitive | All HCC (60) | CP-A, CP-B, liver-confined HCC, prior TACE included | 3.2 (1-6.5) | CP-A : 44 Gy/3 fx CP-B : 40 Gy/5 fx | 27 (2-52) | 90% (2 yr) | 67% (2 yr) |
| Price TR, et al. [14] (2012) | United States, Indiana Univ. | Phase I/II | Definitive/Salvage | All HCC (26) | CP-A, CP-B, ≤3 tumors, single≤6cm, multiple≤sum 6cm, previous Tx allowed | GTV volume: 34 (2-95) cc | CP-A: 48 Gy/3 fx CP-B: 40 Gy/5 fx | 13 (3-42) | CR + PR: 73% | 77% (1 yr) 60% (2 yr) |
| Kang JK, et al. [15] (2012) | Korea, Korea Inst. Of Radiological and Medical Sciences | Phase II | Salvage | All HCC (47) | CP-A, CP-B, inoperable, incomplete response after TACE, PVT allowed | 2.9 (1.3-7.8) | 57 (42-60) Gy/3 fx | 17 (6-38) | 95% (2 yr) | 69% (2 yr) |
| Bujold A, et al. [16] (2013) | Canada, Toronto Univ. | Phase I/II [Trial 1], immediately subsequently phase II [Trial 2] | Definitive | All HCC (102) (trial 1 : 50, trial 2 : 52) | CP-A, PVT allowed, [trial 2] ≤ 5 tumors, <15cm | 7.2 (1.4-23.1) | 36 (24-54) Gy/6 fx | 31 (2-36) | 87% (1 yr) | Median 17 mo |
| Kim JW, et al. [17] (2016) | Korea, Yonsei Cancer Center | Phase I | Definitive/Salvage | All HCC (18) | CP-A, CP-B, ≤3 tumors, single≤5cm, multiple≤sum 6cm, previous Tx allowed | 1.9 (1.0-3.3) | Dose escalation 36-60 Gy/4 fx | 23 (11-38) | Radiologic CR: 89%, 49% (2 yr) | 69% (2 yr) |
Table 2.
| Reference | Institutions | Design | RTAim | Patient number | Indication | Median size (cm) | Dose | Median f/u (range), mo | Local control | Overall survival |
|---|---|---|---|---|---|---|---|---|---|---|
| Choi BO, et al. [18] (2006) | Korea, Catholic University of Korea | Linac | Definitive/Salvage | 20 | CP-A, CP-B, single lesion, previous TACE, PEI, RFA included | 3.8 (2-6.5) | 50 Gy/5 or 10 fx | 23 (3-55) | 100% | 70% (1 yr), 43% (2 yr) |
| Choi BO, et al. [19] (2008) | Korea, Catholic University of Korea | Cyberknife | Definitive/Salvage | 31 (22 : SBRT alone, 9 : TACE + SBRT) | CP-A, CP-B, ≤5cm, PVT surrounding near HCC, previous TACE, PEI, RFA included advanced HCC + PVT -> TACE + SBRT Small HCC -> SBRT alone | 33 (30-39) Gy/3 fx | 11 (2-19) | 100% | 81% (1 yr) | |
| Kwon JH, et al. [20] (2010) | Korea, Catholic University of Korea | Cyberknife | Definitive/Salvage | 42 | CP-A, CP-B, ≤100 cc, unavailable for other local therapies, inoperable, residual lesion after previous Tx, only targets liver parenchyme | 30-39 Gy/ 3 fx | 29 (8-49) | 72% (1 yr), 68% (3 yr) | 93% (1 yr), 59% (3 yr) | |
| Seo YS, et al. [21] (2010) | Korea, Korea Inst. of Radiological and Medical Sciences | Cyberknife | Salvage | 38 | CP-A, CP-B,< 10 cm, all with TACE failure | 33-57 Gy/ 3-4 fx | 15 (3-27) | 66% (2 yr) | 61% (2 yr) | |
| Louis C, et al. [3] (2010) | Belgium, University Hospital Domaine Universitaire Sart Tilman | Cyberknife | Definitive/Salvage | 25 | CP-A, CP-B, PVT allowed, previous TACE, op, RFA, nexavar included | 4.5 (1.8-10) | 45 Gy/3 fx | 13 (1-24) | 95% (1 yr) | 79% (1 yr) |
| Huang WY, et al. [4] (2012) | Taiwan, Tri-Service General Hospital | Cyber knife, matched pair analysis (SBRT vs. other/noTx) | Salvage | 36 | CP-A, CP-B, CP-C, All undergone prior Tx, but tumor progressed, unresectable | 4.4 (1.1-12.3) | 37 (25-48 Gy)/ 4-5 fx | 14 (2-35) | 88% (1 yr), 75% (2 yr) | 64% (2 yr) |
| Honda Y, et al. [22] (2013) | Japan, Hiroshima Univ. | Linac, TACE alone vs. TACE -> SBRT | Salvage | 30 | CP-A, CP-B, solitary, ≤3cm, all prior TACE, no PVT, no EHM | 1.6 (1-3) | 48 Gy/4 fx or 60 Gy/8 fx | 12 (6-38) | 100% | 100% (1 yr), 100% (3 yr) |
| Xi M, et al. [23] (2013) | China, Sun Yat-sen Univ. | VMAT | Definitive/Salvage | 41 | CP-A, all with PVT/IVCT, targets thrombosis, unresectable | 36 (30-48) Gy/6 fx | 10 (4-25) | 95% | 50% (1 yr) | |
| Jang Wl, et al. [24] (2013) | Korea, Korea Inst. of Radiological and Medical Sciences | Cyberknife | Definitive/Salvage | 108 | CP-A, CP-B, unsuitable for other Tx or incomplete TACE | 3 (1-7) | 51 (33-60)/3 fx | 30 (4-81) | 87% (2 yr) | 63% (2 yr) |
| Sanuki N, et al. [25] (2014) | Japan, Ofuna Chuo Hospital | Linac | Definitive/Salvage | 185 | CP-A, CP-B, single, unresectable, no LN mets or EHM | 2.7 (1-5) | CP-A : 40 Gy/5 fx CP-B : 35 Gy/5 fx | 24 (3-80) | 91% (3 yr) | 70% (3 yr) |
| Culleton S, et al. [26] (2014) | Canada, Princess Margaret Cancer Centre | 3D-CRT, IMRT, or VMAT | Definitive/Salvage | 29 | CP-B, <10cm, <5 tumors, life expectancy >3 months, KPS > 60%, unresectable, unsuitable for liver transplantation | 8.66 (4.1-26.6) | median 30 Gy/6 fx | 12 SD, 2 PR 6 intrahepatic PD (outfield) | Median 7.9 months, 32% (1 yr) | |
| BCLC-C | ||||||||||
| Bae SH, et al. [27] (2013) | Korea, Korea Inst. of Radiological and Medical Sciences | Cyberknife | Definitive/Palliative | 35 | BCLC-C, CP-A, CP-B, vascular invasion or extrahepatic metastasis | 45 (30-60) Gy/3-5 fx | 14 (1-44) | 69% (1 yr), 51% (3 yr) | 52% (1 yr), 21% (2 yr) | |
| Huge HCC | ||||||||||
| Que JY, et al. [28] (2014) | Taiwan, Chi Mei Medical Center | Cyberknife | Definitive/Salvage | 22 | CP-A, CP-B, ≥10 cm, ECOG≤2 | 11.36 (10-18) | mainly 40 Gy/5 fx (26-40) | 11.5 (2-46) | 56 % (1 yr), Response rate 86.3% | Median 11 mo, 50% (1 yr) |
| Zhong NB, et al. [29] (2014) | China, Fuzhou General Hospital | Total body gammaray stereotactic radiotherapy system | Salvage | 72 | Incomplete TACE -> SBRT, ≥10cm, ECOG≤2 | 12.6 (10.8-16.5) | 35.7 (33.8-39)/ 6 fx | 18 (4-70) | Response rate 79.1%, low incidence of recur (8.3%) | Median 10.8 mo, 38% (1 yr), 12% (3 yr), 3% (5 yr), significantly higher with tumor encapsulation (56%, 1yr) |
CP, child-pugh; TACE, transcatheter arterial chemoembolization; PEI, percuataneous ethanol injection; RFA, radiofrequency ablation; SBRT, stereotactic body radiotherapy; HCC, hepatocellular carcinoma; PVT, portal vein thrombosis; EHM, extrahepatic metastasis; IVCT, inferior vena cava thrombosis; VMAT, volumetric modulated arc therapy; 3D-CRT, 3D conformal radiotherapy; IMRT, intensity modulated radiotherapy; KPS, Karnofsky performance status; LN, lymph node; SD, stable disease; PR, partial response; PD, progressive disease; ECOG, Eastern Cooperative Oncology Group.
Table 3.
| Reference | Design | Patient number | RT dose | Response rate | Survival | |
|---|---|---|---|---|---|---|
| Prospective studies | ||||||
| Li B, et al. [40] (2003) | Phase II, TACE+RT | 45 | All stage III, KPS ≥70, CP A, B | 45 Gy25 fx -> boost 5.4 Gy/3 fx | CR6, PR 35, SD 4, PD 0 | 1-yr 69%, 2-yr 48%, 3-yr 23%, median 23.5 months |
| Oh D, et al. [41] (2010) | Phase II, TACE+RT | 40 | HCC which failed after 1-2 courses of TACE | Median 54 Gy in 3 Gy/fx | Objective response rate: 63% (CR 9, PR 18), 9 progressions within the irradiated field | 1-yr 72%, 2-yr 46% |
| Leng ZQ, et al. [42] (2000) | Phase III, TACE+RT vs. TACE | 36 vs. 39 | Stage II, III, IVA, KPS ≥65, CP A | 27.6–33.6 Gy to ‘volume including whole live area->11.4–32.4 Gy boost | 1-yr 75% vs. 61%, 2-yr 57% vs. 34% | |
| Zhao MH, et al. [43] (2006) | Phase III, TACE+RT vs. TACE | 49 vs. 47 | T1-2N0M0, KPS ≥70, CP A, PVT (-) | 45-55 Gy | TACE+RT: CR 17, PR 18, PD 5, TACE: CR 8, PR 11, PD 11 | 1-yr 82% vs. 55%, 2-yr 63% vs. 28% |
| Koo JE, et al. [44] (2010) | Phase II, TACE+RT (vs. historical control TACE alone) | 42 vs. 29 | All with IVCT, CP A, B | Median 45 Gy in 2.5-5 Gy/fx (determined by the extent of thrombosis) | Objective response rate: 43% vs. 14% | Median 12 vs. 5 months, 1-yr 48% vs. 17% |
| Choi C, et al. [45] (2014) | Phase II, TACE+RT | 31 | HCC which failed after 1-3 courses of TACE, CP A, B | Median 54 Gy in 1.8-2 Gy/fx | In-field CR 24%, PR 59% at 12 wks Overall CR 10%, PR 52% at 12 wks | 2-yr 61% |
| Retrospective studies | ||||||
| Cheng JC, et al. [46] (2000) | TACE+RT or RT alone | 16, 6 | Stage II-IV, CP A, B, median 10 cm | 46.9 ± 5.9 Gy in 1.8-2 Gy/fx | Only 3 local prgression (area treated with RT) | 1-yr 54%, 2-yr 41%, median 19.2 months |
| Guo WJ, et al. [47] (2003) | TACE+RT vs. TACE | 76 vs. 89 | Stage I-III, KPS ≥70, CP A, B | 1.8–2.0 Gy/fx* 15–28fx | Objective response rate: 47% vs. 28% | 1-yr 64% vs. 40% |
| Li Y, et al. [48] (2003) | TACE+RT vs. TACE | 41 vs. 41 | Size: 3.2-11.5 cm, CP A, B | PTV <216 cm3: 5–8 Gy/fx* 5–12fx, PTV > 216 cm3: 4 Gy/fx* 11-14 fx | TACE+RT: CR 4, PR 23 TACE: CR 1, PR 23 | 1-yr 73% vs. 55% |
| Zeng ZC, et al. [36] (2004) | TACE+RT vs. TACE | 54 vs. 203 | Size>10 cm: overall 31%, CP A, B | 2 Gy/fx* 18-30 fx | CR+PR: 76% vs. 31% | 1-yr 72% vs. 60%, 3-yr 24% vs. 11% |
| Chen WJ, et al. [49] (2014) | TACE+RT vs. TACE | 78 vs. 80 | Objective response rate: 72% vs. 54% | 1-yr 78% vs. 59%, 3-yr 26% vs .16% | ||
| Shim SJ, et al. [37] (2005) | TACE+RT vs. TACE | 38 vs. 35 | Stage III, IV, stage ≥5 cm, CP A, B | 1.8 Gy/fx* 17–33fx | TACE+RT: CR 0, PR 25 | 2-yr 37% vs. 14% (benefit twith large tumors) |
KPS, Karnofsky performance status; CP, child-pugh; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; HCC, hepatocellular carcinoma; PVT, portal vein thrombosis; IVCT, inferior vena cava thrombosis; TACE, transcatheter arterial chemoembolization; RT, radiotherapy; PTV, planning target volume.
Table 4.
| Reference | Institutions | Patient number | MVI site | RT dose | Combined Tx | Response rate | Survival |
|---|---|---|---|---|---|---|---|
| Prospective studies | |||||||
| Yamada K, et al. [53] (2003) | Japan, Kobe Univ. | 19 | All: 1st PVTT | 60 Gy/30 fx | TACE in all pts | CR+PR 58% | Median: 7 months, 1-yr: 40.6% |
| Han KH, et al. [54] (2008) | Korea, Yonsei Cancer Center | 40 | Main: 13 | 45 Gy/25 fx | CCRT with IACTx followed by IACTx | CR 0 PR 45% SD 23% | Median 13.1 months, 1-yr 57.6% |
| 1st: 27 | |||||||
| Shirai S, et al. [55] (2010) | Japan, Wakayama Medical Univ. | 19 | Main: 12 | 45 Gy/18 fx | TACE in all pts | For PVTT: CR 0% PR 37% SD 53% | Median 10.3 months, 1-yr 47% |
| 1st: 7 | |||||||
| Koo JE, et al. [44] (2010) | Korea, AMC | 42 | IVCTT 42 (co-existence of PVTT in 19 pts) | 28-50 Gy (median 45 Gy) | TACE in all pts | CR 14% PR 29% SD 29% | Median 11.7 months, 1-yr 47.7% |
| Chuma M, et al. [56] (2011) | Japan, Hokkaido Univ. | 20 | All: main, 1st PVTT | 30-48 Gy/7-16 fx | CCRT | CR 0% PR 35% SD 45% | Median 12 months, 1-yr n-s |
| Park MS, et al. [57] (2013) | Korea, Yonsei Cancer Center | 30 | All: 1st PVTT | 45 Gy/25 fx | TACE followed by CCRT with IACTx | CR 0% PR 30% SD 40% | Median 9.8 months, 1-yr n-s |
| Retrospective studies | |||||||
| Katamura Y, et al. [58] (2009) | Japan, Hiroshima Univ. | 16 | All: main, 1st PVTT | 30,39,45 Gy/10,13,15 fx | CCRT with IACTx (5FU+IFN) in all pts | For PVTT: CR 19% PR 56% SD 25% | Median 7.5 months, 1-yr n-s |
| Yu JI, et al. [38] (2011) | Korea, SMC | 281 | SMV 13, Main PVTT 114, Both 1st 4, 1st PVTT 150 | 30-54 Gy, BED 39-70.2 Gy10 | TACE in 90%, Others: reRT, RFA, sorafenib, op | CR 4% PR 50% SD 26% | Median 11.6 months, 1-yr 48.1% |
| Yoon SM, et al. [59] (2012) | Korea, AMC | 412 | Main or 1st PVTT 300, other PVTT 212 | 21-60 Gy (median 40 Gy), BED 27.3-78 Gy10 (median 51.75 Gy10) | TACE in almost all pts | HCC: CR 4%, PR 24%, SD 8% PVTT: CR 7%, PR 33%, SD 46% | Median 10.6 months, 1-yr 42.5% |
| Hou JZ, et al. [60] (2012) | China, Fudan Univ. | 181 | Main PVTT 87 | 30-60 Gy (median 50 Gy) | TACE, as possible as | PVTT: CR 21%, PR 33%, SD 39% IVCTT: CR 55%, PR 27%, SD 18% | Median: main PVTT 7.4 months, branch 10.2 months, IVC 17.4 months, both 8.5 months 1-yr: main PVTT 36%, branch 49%, IVC 68%, both 42% |
| Branch PV 39 | |||||||
| IVC 37 | |||||||
| IVC+PV 18 | |||||||
| Tang QH, et al. [61] (2013) | China, 2 institutions | 185 | Main 49 | 30-52 Gy (median 40 Gy), BED 39-75 Gy10 | TACE in all pts | N-S | Median 12.3 months, 1-yr 51.6% |
| 1st 64 | |||||||
| 2nd 72 | |||||||
| Cho JY, et al. [62] (2014) | Korea, SMC | 67 | MVI 64 (Main PVTT 17) | 30-45 Gy/10-22 fx BED 39-65.25 Gy10 (median 47.25 Gy10) | TACE in all pts | N-S | Median 14.1 months, 1-yr n-s |
| Tanaka Y, et al. [63] (2014) | Japan, Kitasato Univ. | 67 | 1st PVTT 25 | 30-56 Gy | N-S | CR 7% PR 37% SD 24% PVTT RR 45%; HV RR 59% Both20% | Median 9.4 months, 1-yr 39% |
| Main PVTT 15 | |||||||
| 1st HV 4 | |||||||
| IVC 13 | |||||||
| PVTT+HVI 10 | |||||||
| Yu JI, et al. [64] (2014) | Korea, Multicenter | 994 | PVTT 994 | 7.2-66 Gy (median 45 Gy), BED 8.5-137.7 Gy10 (median 56.25 Gy10) | TACE in almost all pts | PVTT CR 5% PR 41% SD 41% | Median 9.2 months, 1-yr 40% |
| -main 497 | |||||||
| - others497 | |||||||
| Duan F, et al. [65] (2015) | China, General Hospital of Chinese People’s Liberation Army | 11 | IVC and RA 11 | 60 Gy/30 fx | TACE in all pts | IVC & RA: CR 100% HCC: CR 18%, PR 55% SD 18% | Median 21 months, 1-yr 54.5% |
| Yu JI, et al. [66] (2016) | Korea, SMC & AMC | 508 | PVTT 508 | BED 27.3-78 Gy10 (median 54.6 Gy10) for training, 15.6-71.5 Gy10 (median 50.4 Gy10) for validation | TACE in almost all pts | PVTT Training: CR 10%, PR 41%, SD 39% | N-S |
| -main 217 | Validation: CR 10%, PR 54%, SD 24% | ||||||
| -others 291 | |||||||
| Im JH, et al. [67] (2017) | Korea, Multicenter | 985 | All: main, 1st PVTT | 12-66 Gy (median 45 Gy) in 1.8-17 Gy | TACE/TACI 80%, HAIC 16%, TACE/TACI+HAIC 4%, no Tx 33% | PVTT: CR 6%, PR 46%, SD 40%, PD 8%, HCC: CR 5%, PR 48%, SD 39%, PD 8% | Median 20 months, 1-yr 43%, 2-yr 22% |
PVTT, portal vein tumor thrombosis; IVCTT, inferior vena cava tumor thrombosis; HV, hepatic vein; RA, right atrium; TACE, transcatheter arterial chemoembolization; TACI, transcatheter arterial chemoinfusion; CR, complete response; PR, partial response; SD, stable disease; CCRT, concurrent chemoradiotherapy; IACTx, intraarterial chemotherapy; BED, biologically equivalent dose; RR, response rate.
Table 5.
| Reference | Institutions | Design | Patient number | Indication | Median size (cm) | Dose | Median f/u (range), mo | Local control | Overall survival | Adverse events |
|---|---|---|---|---|---|---|---|---|---|---|
| Chiba T, et al. [88] (2005) | Japan, Tsukuba | Retrospective | 162 | <3:27%, 3-5:56%, 5<:17% | 72 GyE/16 fx, 78GyE/ 20 fx, 84 GyE/24 fx, 50 GyE/10 fx | 87% (5-yr) | 24% (5-yr) | Very few acute reactions, 5 ≥Gr 2 toxicity | ||
| Fukuda K, et al. [95] (2017) | Japan, Tsukuba | Retrospective | 129 | CP A or B, ECOG 0-2, previously untreated | 3.9 | 77 GyE/35 fxl 72.6 GyE/22 fx, 66 GyE/10 fx (according to location) | 55 (43-67) | 94% (5-yr) | 69% (0/A stage), 66% (B stage), 25% (C stage) (5-yr) | No ≥Gr 2 late toxicity, radiation dermatitis was common, but no ≥Gr 3 toxicity |
| Bush DA, et al. [89] (2011) | United States, Loma Linda University | Phase I | 76 | CP A, B, or C, All with cirrhosis | 5.5 | 63 GyE/15 fx | Local control: 60/76 | Median PFS 36 months | 5 Gr2 toxicities, no significant overall deterioration of liver function | |
| Hong TS, et al. [91] (2014) | United States, MGH | Phase I | 15 | CP A or B | CTV: median 124 (20-581) cc | 45-75 GyE/15 fx | 69 (for survivors) | No infield recurrence, Marginal recurrence: 1/15 | 33% (3-yr), Median 12 months | 2 Gr 3 bilirubinemia, 1 Gr 3 gastrointestinal bleed, 1 Gr 5 stomach perforation |
| Kim TH, et al. [93] (2015) | Korea, NCC | Phase I | 27 | ≤6cm, CP A or B | 3.2 for dose level I, 2.3 for dose level II, 2.5 for dose level III | 60 GyE/20 fx, 66 GyE/22 fx, 72 GyE/24 fx | 71-83% (3-yr), CR: 63% for dose level I, 57% for dose level II, 100% for dose level III | 42% (5-yr), Median 38 months | No ≥Gr 2 late toxicity | |
| Kawashima M, et al. [94] (2005) | Japan, NCC Hospital East | Phase II | 30 | All with cirrhosis, R15 ≥ 15%, CP A or B | 4.5 | 76 GyE/20 fx | 31 | 96% (2-yr) | 66% (2-yr) | 8 hepatic insufficiencies, no hepatic insufficiency when ICG R15 <20% |
| Fukumitsu N, et al. [95] (2009) | Japan, Tsukuba | Phase II | 51 | CP A or B | 2.8 | 66 GyE/10 fx | 34 | 88% (5-yr) | 39% (5-yr) | 8 CPS deteriorations, late: 3 rib fractures, 1 Gr 3 radiation pneumonitis |
| Hong TS, et al. [92] (2016) | United States, MGH | Phase II | 49 | CP A or B | 5 | 58.05-67.5 GyE/15 fx | 19.5 (for survivors) | 95% (2-yr) | 63% (2-yr), Median 49.9 months | 4 Gr 3 toxicities |
| PVTT (+) | ||||||||||
| Lee SU, et al. [97] (2014) | Korea, NCC | Retrospective | 27 | PVTT(+), CP A or B | 7 | 50-66 GyE/20-22 fx | 13 (2-52) | Objective response rate: 63% for primary tumor, 56% for PVTT, local recurrence: 9/27 | 33% (2-yr), Median 13 months | No ≥Gr 3 toxicity |
| Sugahara S, et al. [98] (2009) | Japan, Tsukuba | Retrospective | 35 | PVTT(+), CP A or B | 6 | 72.6 GyE/22 fx | 21 (2-88) | CR: 8/35, PR: 21/35, local PFS: 20% (5-yr) | Median 22 months, 48% (5-yr) | 3 ≥Gr 3 acute toxicity, No ≥Gr 3 late toxicity |
| Hata M, et al. [99] (2005) | Japan, Tsukuba | Retrospective | 12 | PVTT(+), CP A or B | 6 | 50-72 GyE/10-22 fx | 28 (4-88) | CR: 2/12, PR: 10/12 | 58% (5-yr) | Mild acute toxicities, low late toxicity rate (3 mild telangiectasia) |
Abbreviations
REFERENCES
- TOOLS
-
METRICS

- ORCID iDs
-
Jinsil Seong

https://orcid.org/0000-0003-1794-5951 - Related articles
-
Severity of microvascular invasion does matter in hepatocellular carcinoma prognosis
Carbon Ion Radiotherapy in the Treatment of Hepatocellular Carcinoma2023 October;29(4)
Recent advances in the management of hepatocellular carcinoma2024 January;30(1)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



