| Clin Mol Hepatol > Volume 30(1); 2024 > Article |
|
ABSTRACT
ACKNOWLEDGMENTS
FOOTNOTES
Figure 1.

Figure 2.
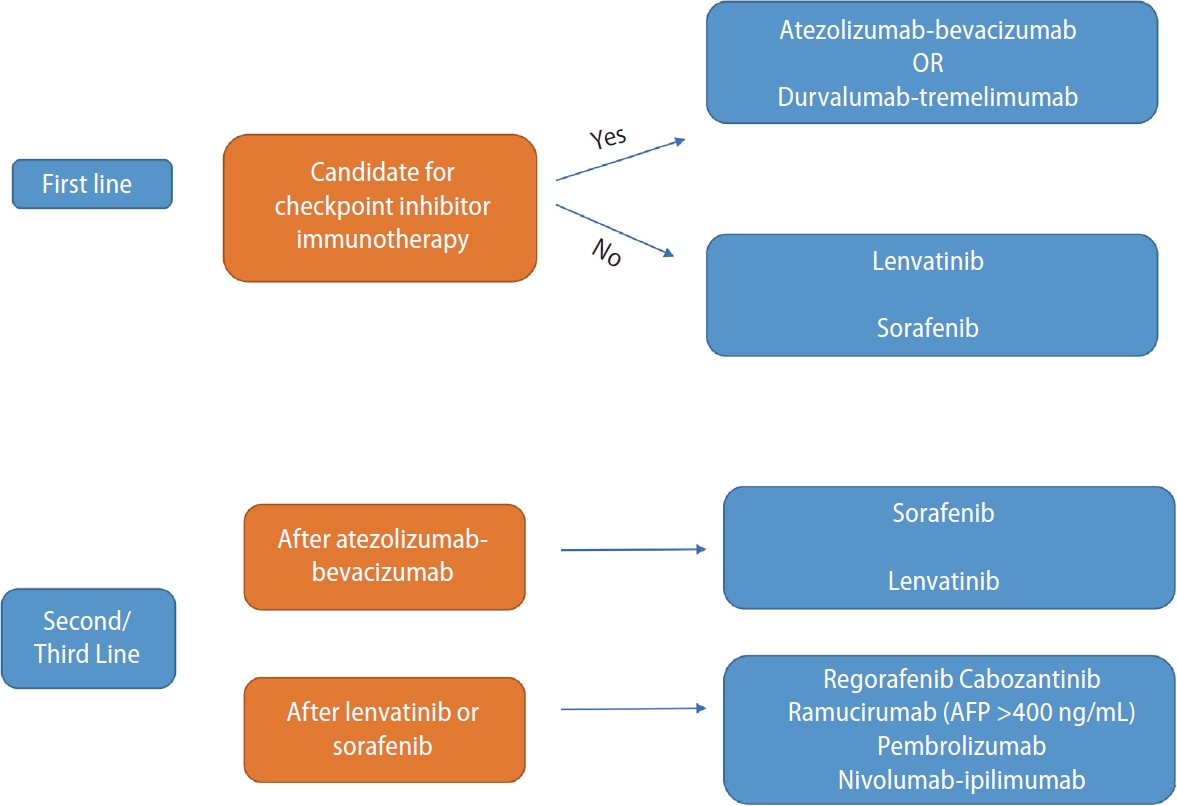
Table 1.
| Trial (reference) | Sample size (n) | Inclusion criteria | Phase and comparator | Primary endpoint(s) | Results |
|---|---|---|---|---|---|
| SHARP [11] | 602 | Patients with advanced HCC without prior systemic therapy, with ECOG PS 0-2 and Child- Pugh liver function class A | 3 | Overall survival | Median OS 10.7 vs. 7.9 months (HR 0.69; P<0.001) |
| Sorafenib 400 mg of twice daily or placebo | Time to symptomatic progression | Median TTP 4.1 vs. 4.9 months (HR 1.08; P=0.77) | |||
| REFLECT [12] | 1,492 | Unresectable HCC with measurable target lesions, BCLC stage B or C, Child-Pugh Class A, and ECOG 0-1 | 3 | Overall survival | Median OS 13.6 vs. 12.3 month (HR 0.92) |
| Lenvatinib 8 mg or 12 mg based on body weight or sorafenib 400 mg twice daily | |||||
| RESORCE [15] | 843 | Patients with BCLC stage B or C HCC not eligible for local treatments with documented radiologic progression during sorafenib treatment, with Child-Pugh class A Liver function. | 3 | Overall survival, analyzedby intention to treat | Median OS 10.6 vs. 7.8 months (HR 0.63; P<0.0001) |
| Regorafenib 160 mg daily or placebo once daily for the first 3 week of each 4-week cycle | |||||
| CELESTIAL [16] | 707 | HCC not amenable to curative treatment with Child-Pugh class A liver function with up to 2 lines of prior treatment for HCC (including sorafenib) with ECOG PS 0-1 | 3 | Overall survival | Median OS 10.2 vs. 8.0 months (HR 0.76; P=0.005) |
| Cabozantinib 60 mg daily or matching placebo | |||||
| REACH-2 [17] | 292 | Patients with BCLC stage B or C HCC treated with prior sorafenib, with AFP ≥400 ng/mL | 3 | Overall survival | Median OS 8.5 vs. 7.3 months (HR 0.71; P=0.0199) |
| Ramucirumab 8 mg/kg or placebo every 14 days |
Table 2.
| Trial (reference) | Sample size (n) | Inclusion criteria | Phase and comparator | Primary endpoint(s) | Results |
|---|---|---|---|---|---|
| KEYNOTE-224 [20] | 104 | Patients with BCLC stage B or C HCC who were intolerant to or progressed on sorafenib, that was not amenable to or refractory to curative treatment approach, with ECOG 0–1, and Child-Pugh class A liver function | 2 | ORR per RECIST v1.1 [27] | ORR 18% (95% CI 11–26%) |
| Pembrolizumab 200 mg every 3 weeks for up to 35 cycles | CR 1%, PR 16%, SD 44% | ||||
| CheckMate 040 [19] | 262 | Advanced HCC who progressed on at least one prior line of treatment including sorafenib, with Child-Pugh B7 or A and ECOG 0–1 | I/II | ORR (dose-expansion phase) | ORR 20% (95% CI 15–26) |
| Nivolumab every 2 weeks | CR 1%, PR 18%, SD 45% | ||||
| CheckMate 040 [26] | 148 | HCC not eligible for curative treatment with Child-Pugh class A liver function, ECOG PS 0–1 | 3 | Safety and tolerability, and ORR | ORR 32% (arm A), 27% (arm B), and 29% (arm C) |
| A: Nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks for 4 doses | |||||
| B: nivolumab 3 mg/kg plus ipilimumab 1 mg/kg every 3 weeks | |||||
| C: nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/ kg every 6 weeks | |||||
| CheckMate 459 [21] | 743 | Advanced HCC not eligible for locoregional therapies, Child-Pugh class A liver function, ECOG 0–1, with no prior systemic therapy | 3 | OS | Median OS 16.4 vs. 14.7 months (HR 0.85; P=0.075) |
| Nivolumab 240 mg every 2 weeks or sorafenib 400 mg twice daily | |||||
| CheckMate 240 [22] | 413 | HCC with progression or intolerance to sorafenib treatment, BCLC stage B or C disease, Child-Pugh class A liver disease, ECOG 0–1 | 3 | OS | Median OS 13.9 vs. 10.6 months (HR 0.781; P=0.0238) |
| Pembrolizumab 200 mg every 3 weeks or placebo for up to 35 cycles | PFS | Median PFS 3.0 vs. 4.1 months (HR 0.775; P=0.0186) | |||
| IMBrave150 [13, 24] | 336 | Locally advanced, metastatic, or unresectable HCC with no prior systemic therapy, that was not amenable to curative or locoregional therapies or had progressed thereafter, with Child-Pugh liver function A and ECOG 0–1 | 3 | OS | Median OS 19.2 vs. 13.4 months (HR 0.66; P=0.0009) |
| Atezolizumab 1,200 mg plus bevacizumab 15 mg/kg every 3 weeks or sorafenib 400 mg twice daily | Progression-free survival | Median PFS 6.8 vs. 4.3 months (HR 0.59; P≤0.001) | |||
| HIMALAYA [14] | 1,171 | HCC with no prior systemic therapy that was ineligible for locoregional therapy with ECOG PS 0–1, Child-Pugh class A liver function | 3 | OS | Median OS 16.43 (STRIDE) vs. 13.77 (sorafenib) months (HR 0.78; P=0.0035) |
| Tremelimumab 300 mg for one dose plus durvalumab 1,500 mg every 4 weeks (STRIDE) or tremelimumab 75 mg every 4 weeks for 4 doses plus durvalumab 1500 mg every 4 weeks (T75+D) or durvalumab 1,500 mg every 4 weeks or sorafenib 400 mg twice daily |
ICI, immune checkpoint inhibitors; HCC, hepatocellular carcinoma; BCLC, Barcelona Clinic Liver Cancer; EECOG PS, Eastern Cooperative Oncology Group Performance status; CR, complete response; PR, partial response; SD, standard deviation; CI, confidence interval; OS, overall survival; HR, hazard ratio.
Abbreviations
REFERENCES
-
METRICS

- ORCID iDs
-
Kamya Sankar

https://orcid.org/0000-0001-8636-8479Ju Dong Yang

https://orcid.org/0000-0001-7834-9825 - Related articles
-
Carbon Ion Radiotherapy in the Treatment of Hepatocellular Carcinoma2023 October;29(4)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



