| Clin Mol Hepatol > Volume 29(4); 2023 > Article |
|
ABSTRACT
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related death, and external beam radiation therapy has emerged as a promising approach for managing HCC. Proton beam therapy (PBT) offers dosimetric advantages over X-ray therapy, with superior physical properties known as the Bragg peak. PBT holds promise for reducing hepatotoxicity and allowing safe dose-escalation to the tumor. It has been tried in various clinical conditions and has shown promising local tumor control and survival outcomes. A recent phase III trial demonstrated the non-inferiority of PBT in local tumor control compared to current standard radiofrequency ablation in early-stage HCC. PBT also tended to show more favorable outcomes compared to transarterial chemoembolization in the intermediate stage, and has proven effective in-field disease control and safe toxicity profiles in advanced HCC. In this review, we discuss the rationale, clinical studies, optimal indication, and future directions of PBT in HCC treatment.
Hepatocellular carcinoma (HCC) is the most common primary malignancy arising in the liver and is increasing in incidence with significant impacts on morbidity and mortality [1]. While surgical management remains the primary treatment option, the majority of patients are not appropriate candidates due to advanced disease at diagnosis or poor expected postoperative liver function or surgical morbidity. This renders alternative local treatments critical for the long-term management of these patients in a variety of settings. Nonsurgical treatment options include percutaneous ablation, transarterial chemoembolization (TACE), selective internal radiotherapy (SIRT), and external beam radiation therapy (EBRT). EBRT modalities include 3D conformal radiotherapy (3D-CRT), intensity-modulated radiation therapy (IMRT), volumetric-modulated arc therapy (VMAT), stereotactic body radiation therapy (SBRT), proton beam therapy (PBT), and carbon ion radiotherapy (CIRT). Because key risk factors for the development of HCC include cirrhosis of any cause and hepatitis B or C virus infections, EBRT has had a very limited role in the treatment of HCC in patients whose livers are mostly cirrhotic or poorly functioning, as these patients are most vulnerable to radiation-induced liver disease (RILD) [2,3]. With recent advances in EBRT technology, photon EBRT such as IMRT demonstrated clinical efficacy without increasing RILD in the management of HCC, and the role of PBT has emerged as a powerful technique with its superior physical property in ablative dose delivery to tumors while sparing the uninvolved liver and other nearby critical organs. Here, we review the current evidences and potential roles of PBT according to the Barcelona Clinic Liver Cancer (BCLC) staging classifications [4] in the management of HCC
The liver is one of the important radiation dose-limiting organs during EBRT, with RILD risk associated with the irradiated liver volume [5-7]. Thus, minimizing the radiation dose to the remaining normal liver during EBRT for HCC is crucial. Conceptually, more sophisticated EBRT techniques, including IMRT, VMAT, and PBT, may improve tumor control in HCC patients by delivering a high radiation dose to the tumor while minimizing the dose to the remaining normal liver, thereby minimizing impairment of the remaining hepatic reserve.
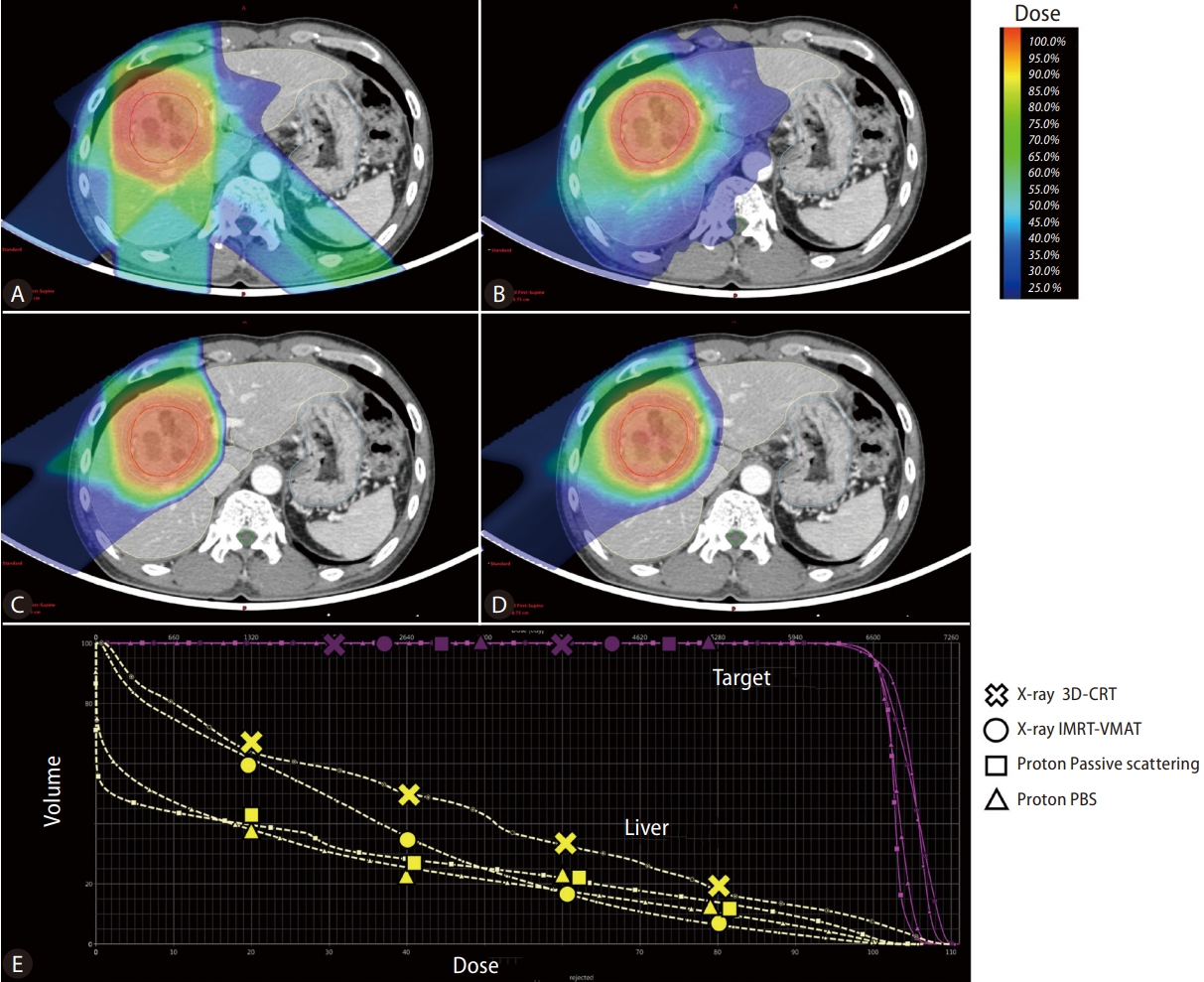
Regarding the physical characteristic, the X-ray dose delivered decreases gradually along the beam path with increasing beam depth [8]. Thus, an exit dose is inevitably delivered to adjacent normal tissues, and even IMRT or VMAT cannot avoid low-dose delivery at the distal area of the beam path. In contrast, a proton beam has a finite range of energy deposition and loses most of its energy within a very short distance at the end of the beam range. This results in a sharp rise and fall in energy absorption, known as the Bragg peak. Therefore, PBT has been considered to have superior physical properties compared to other X-ray-based EBRT techniques, delivering a high radiation dose to the tumor while minimizing the radiation dose delivered to the remaining normal liver, thereby minimizing impairment of the remaining hepatic reserve. Figure 1 presents the radiation dose distribution of various treatment plans using X-ray and PBT for representative 5.7 cm sized HCC case in segment 8. PBT showed the advantage of less radiation exposure in the remaining normal liver, especially in the low-dose area. Regarding the effect of PBT according to tumor size, previous dosimetric analyses have demonstrated that the larger the tumor size, the greater the benefit of PBT in decreasing the risk of RILD [9,10].
Several dosimetric comparison studies have demonstrated the dose volumetric benefits of PBT compared to 3D-CRT and/or IMRT for HCC. Li et al. [11] found that PBT reduced the mean liver dose (Dmean), the fractional volume of the liver receiving doses greater or equal to 10 Gy (V10), 20 Gy (V20), 30 Gy (V30) and better spared non-liver organ-at-risks (OAR) (stomach and kidney) than 3D-CRT or IMRT. Wang et al. [12] demonstrated similar results, with significant reductions in the V30 of the remaining normal liver and the Dmean, as well as reduced stomach, duodenum, heart, and spinal cord by PBT compared to IMRT. Kim et al. [13] compared dose-volume histogram data among helical-IMRT (H-IMRT), VMAT, and PBT for HCC and found that PBT provided equal planning target volume (PTV) tumor coverage, conformity index, and homogeneity index values and significantly better sparing of the liver (Dmean and V5 to V35 for remaining normal liver) and non-liver OARs (D2 cm3 of the stomach and spinal cord) compared to H-IMRT and VMAT. Even though the difference between PBT and either H-IMRT or VMAT in the irradiated volumes of the remaining normal liver at higher doses (from V45 to V55) may not have been clinically significant (less than 3%), PBT significantly reduced the irradiated liver volume at dose levels below V35 (about 50% of the prescribed dose) [13]. These data suggest that PBT may be superior to other EBRT modalities, including 3D-CRT, H-IMRT, and VMAT, in reducing the risk of RILD, and it may also have an advantage during dose escalation.
Numerous published data demonstrating the efficacy of PBT for HCC have utilized passive scattering (PS)-PBT treatment techniques. Pencil beam scanning (PBS)-PBT may provide more conformal dose distribution and superior normal tissue sparing by the intensity modulation of PBT, enabling improved dose optimization and conformity along the proximal edge compared to PS-PBT [14,15]. PBS-PBT is considered more sensitive to organ motion with respiration than passive scattering due to the interplay effect [15]. Recently, published retrospective series demonstrated the feasibility, safety, and efficacy of PBS-PBT for treating HCC. Although only small retrospective series have been reported, most studies achieved high local tumor control (around 95% at one year) and low toxicity profiles [16,17]. Yoo et al. [18] compared the outcomes of HCC patients treated with either PS-PBT or PBS-PBT. After propensity score matching, they revealed no difference in toxicity, tumor control, or survival between patients treated with PS-PBT or PBS-PBT [18]. As a majority of newer PBT centers have PBS-PBT capability, an increase in data supporting PBS-PT for HCC treatment is anticipated.
In selected cases, PBT can be the optimal EBRT technique for delivering a second course of radiation with a curative dose to a previously irradiated liver by preserving the remaining healthy liver and the adjacent critical abdominal organs [19]. Early reports of Hashimoto et al. [20] published in 2006 demonstrated the safety and feasibility of proton re-irradiation for recurrent HCC patients (n=27), and treatment-related toxicities of grade 3 or higher were observed in about 18% (n=5), of which 2 developed acute hepatic failure and the remaining 3 had late injuries (1 rib fracture and 1 bile duct stenosis). McDuff et al. [21] (n=49) reported the more recent experiences confirming the safety and efficacy of proton re-irradiation for liver malignancies with the median follow-up of 10.5 months since re-irradiation. With regard to toxicity, only 2 patients (4.1%) experienced grade 3 toxicity of radiation-induced liver disease after reirradiation. Oshiro et al. [22] retrospectively assessed 83 patients who received liver re-irradiation with protons, including 15 patients treated with three or more definitive EBRT courses. For most patients, repetitive PBT is the only possible treatment option because of the lack of other local therapeutic options. Patients were treated with similar PBT doses of 70 gray-equivalent (GyE) at re-irradiation, following an initial course of 71 GyE. The most commonly used dose schemes were 72.6 GyE in 22 fractions (33%), 66 GyE in 10 fractions (33%), and 74 Gy in 27 fractions (19%) (mean dose per fraction 3.3 GyE). Second-course planning considerations generally included maintaining a normal liver volume of more than 500 mL. This second course of PBT with definitive doses resulted in a median OS of 61 months from the time of the first treatment course, with no severe radiation-induced liver dysfunction or other acute toxicities [22].
While PBT has been established as an acceptable local therapeutic option for early-to-advanced HCC, there still is a lack of high-quality evidence to guide recommendations for when proton therapy should be clearly preferred over photon therapy. Cheng et al. [23] compared 110 HCC patients treated with either photon EBRT (n=55) or PBT (n=55) with curative intent after propensity matching. About half of the patients had vascular invasion. Cox regression analysis revealed a significant survival benefit (P=0.032, hazard ratio [HR]=0.56, 95% confidence interval [CI]: 0.33ŌĆō0.96) and lower risk of RILD (11.8% vs. 36%, P=0.004) in the PBT group compared to the photon group [23]. Qi et al. [24] performed a meta-analysis to compare charged particle therapy versus photon therapy for HCC patients. The pooled OS was significantly higher for charged particle therapy than for conventional photon EBRT with improvements in progression-free survival and local control, while comparable efficacy was found between charged particle therapy and SBRT in OS, progression-free survival and local control. High-grade acute and late toxicity associated with charged particle therapy was lower than that of conventional photon EBRT and SBRT [24]. Hasan et al. [25] compared PBT (n=71) with SBRT (n=918) in stage I-II HCC patients in the National Cancer Database. The results showed that PBT was independently associated with longer survival than SBRT (HR=0.48, 95% CI: 0.29ŌĆō0.78), despite being delivered to HCC patients with multiple poor prognostic factors [25]. NRG GI003 (NCT03186898) is an ongoing randomized trial to compare PBT versus conventional photon EBRT for HCC.
CIRT, a type of particle beam therapy, is known to have potential advantages with higher radiobiological effectiveness (RBE), offering therapeutic benefits in hypoxic or radioresistant tumor cells [26]. Thus, CIRT has broadened its clinical application and was recently attempted in HCC. Despite the potential benefits of CIRT with high RBE values, the clinical outcome of CIRT appears to be similar to that of PBT, with about 80ŌĆō90% local tumor control and 50% OS at three years [27,28].
From a treatment planning perspective, dosimetric studies suggest that PBT is advantageous for normal liver-sparing in patients with tumors >3 cm in specific locations or for larger tumors (>5 cm) in patients with good baseline liver function [9,29,30]. For patients with deteriorated liver function, the indication for PBT should be more generous. Additional insight can be gained from the consensus report from the Miami Liver Proton Therapy Conference held in 2019 [31]. This expert panel recommended that proton therapy be strongly considered in the following scenarios: i) normal liver dose constraints cannot be met with photon therapy; ii) Child-Pugh B or greater cirrhosis based on data showing that low doses to the normal liver are associated with hepatotoxicity [32] and favorable outcomes with PBT [33]; iii) larger tumor size; iv) smaller uninvolved liver volume (e.g., <800 cm3), common in more severely cirrhotic patients and after partial liver resection; v) high tumor-to-liver ratio; vi) multiple number of tumors; and vii) prior radiation therapy to the liver.
Early-to-intermediate-stage HCC encompasses the largest subgroup of patients with HCC. The recommended early-stage treatments are surgical resection, ablation, and transplantation, and the expected five-year overall survival (OS) rate ranges from 50 to 70% [4,34-36]. The standard therapeutic approach for patients with intermediate-stage disease is TACE, and the expected median survival time is about 30 months [4,37]. PBT demonstrated an excellent local control rate reaching 85ŌĆō95% and a comparable OS rate of more than 50% at 3ŌĆō5 years after PBT in patients who received the current standard treatment for early-to-intermediate-stage HCC (Table 1) [27,38-50].
PBT may have several advantages, such as non-invasiveness, use in locations unsuitable for other therapy, and non-echogenicity tumor compared to other local treatments, such as surgical resection and percutaneous ablative therapy, including radiofrequency ablation (RFA). The first phase 3 randomized controlled trial compared PBT and RFA in small recurrent/residual HCC (size <3 cm, number Ōēż2) and demonstrated that the local control effect of PBT was not inferior to that of RFA (PBT 86.5% vs. RFA 78.3% at 4-year, P=0.114) in HCC patients [49]. Crossover was allowed if the assigned treatment was technically infeasible, and PBT showed better feasibility than RFA with significantly lower crossover rate (8.3% vs. 26.4%, P=0.004). The most common treatment-related toxicity was radiation pneumonitis (32.5%) for PBT and increased alanine aminotransferase levels (96.4%) and abdominal pain (30.4%) for RFA, respectively. PBT was tolerable and safe, consistent with the known profile. The associated good feasibility and comparable clinical outcomes suggest that PBT may be a promising treatment option for small HCCs. TACE is a common treatment option for intermediate-stage or multiple HCCs, but local tumor control is often disturbed by the complex arterial blood supply of the tumor, such as collaterals [51]. In general, tumor control by PBT is not compromised by the complexity of the tumor blood supply. Bush et al. [52] recently reported their final results of a prospective randomized clinical trial comparing PBT with TACE in unresectable HCC. PBT was associated with better PFS and LC compared to TACE and even associated with fewer posttreatment hospitalization days, and reduced cost of treatment.
PBT seems to have excellent long-term local tumor control in treatment-na├»ve HCC patients, ranging from 87% to 94%, with an OS rate ranging from 66 to 69%, which is comparable to other recommended first-line treatments. Fukuda et al. [53] reported 5-year outcomes in 129 treatment-na├»ve HCC patients after delivering a total of 66.0ŌĆō77.0 GyE of PBT in 10ŌĆō35 fractions. The 5-year local tumor control and OS rates were 94% and 69% for patients with 0/A stage disease (n=9/21) and 87% and 66% for patients with B-stage disease (n=34), respectively. Kim et al. [54] reported the clinical outcomes of 46 treatment-na├»ve HCC patients treated with PBT and showed similar results, with a 5-year freedom from local progression rate of 92.7% and OS rate of 69.2%.
Since most PBT data from early-to-intermediate-stage HCC were obtained from patients with recurrent or residual HCC, the recently developed 2022 Korean Liver Cancer Association-National Cancer Center Korea practice guidelines for the management of HCC described the role of EBRT including PBT as an effective local treatment option limited to small recurrent HCC [55]. However, PBT has demonstrated similar efficacy in terms of local control, survival, and toxicity in treatment-naïve HCC patients compared to those with recurrent HCC, as the data mentioned above. Hence, we may have to assume that PBT has a potential role as a first-line therapy for treatment-naïve HCC patients as well as those with recurrent or residual HCC. Overall, the data suggest that PBT could be an effective alternative or complementary local treatment for early-to-intermediate-stage HCC where other local treatments, such as surgical resection, ablative therapy, and TACE, might be unsuitable or ineffective, and could be a potential first-line treatment in treatment-naïve HCC patients. In addition, PBT was an effective way to increase the chances of curing localized HCC in liver transplantation candidates as definitive or bridging therapy while they wait for transplantation with less morbidity compared to TACE [52].
The recommended treatment for advanced-stage HCC, BCLC C, is systemic therapy, such as sorafenib, with an expected median survival time of about 10 months. A recent randomized trial comparing atezolizumab plus bevacizumab to sorafenib showed that the combination of atezolizumab and bevacizumab significantly improved objective responses and OS compared to sorafenib [56]. However, the objective response was still around 20ŌĆō30%, and relatively selected patients, such as only those in the Child-Pugh A class, participated and more than 50% of the patients experienced serious adverse events (grade 3ŌĆō4 toxicity) [56].
Nevertheless, local treatments, such as PBT, still may have a role in advanced HCC, especially in HCC with vascular invasion. A randomized controlled study compared combination of EBRT plus TACE with sorafenib in HCC with macroscopic vascular invasion, and demonstrated superior efficacy of EBRT plus TACE with survival improvement compared to systemic therapy [57]. In neoadjuvant setting, EBRT provided significantly better postoperative survival outcomes in patients with resectable HCC and PVTT [58]. Table 2 summarizes the results of previously published PBT data for HCC patients with vascular invasion. The reported median survival time of those patients after PBT was 16 to 22 months [27,45,47,59-63]. These PBT outcomes were better than the outcomes expected of systemic treatment. In particular, the response rate was remarkably high, at up to 60ŌĆō100%. After PBT, recanalization of the portal vein followed by the restoration of liver function might be a possible reason for the improvement in survival. In addition, treatment-related toxicity was relatively mild compared to systemic treatment, with Ōēźgrade 3 toxicity rates of 0ŌĆō13% after PBT. Among EBRT modalities for the treatment of HCC with macrovascular invasion, a recent meta-analysis showed a significantly better OS rate in the PBT group, which was 61%, 45%, and 45% in the PBT, conventional EBRT, and SBRT groups, respectively (P<0.05 for each comparison) [64]. For future perspectives, the combination of PBT with immune-checkpoint inhibitors such as anti-PD1/PDL1 is thought to be a promising treatment option, showing improved progression-free survival up to 27 months with curative intent treatment in a single retrospective study [63].
In conclusion, since PBT has a dosimetric benefit through superior physical properties, it has been thought to have advantages over conventional EBRT, as well as other local therapies, in the management of patients with HCC. Numerous clinical studies have demonstrated PBT as a highly effective local therapeutic option for early-to-advanced HCC, with favorable survival outcomes and a low toxicity rate. PBT is also being used successfully in challenging clinical conditions, such as major vascular invasion and re-irradiation cases. Nevertheless, the clinical evidence for PBT in HCC has been considered insufficient so far, as most studies may have involved inherent selection biases of a retrospective nature and biases towards new technologies, and it contains relatively few patients for high-level evidence. Hence, further research is warranted, and some studies are currently underway, including comparisons of PBT with other treatment modalities or combinations, as well as other types of EBRT, such as photon EBRT or CIRT. For instance, comparisons of PBT versus ablative therapy in patients with treatment-naïve early-stage HCC, the efficacy of combinations of PBT and TACE or SIRT in intermediate-stage disease, or combinations of PBT and systemic therapy in advanced-stage disease will be promising research topics for future clinical trials and will enhance evidence-based clinical guidance and improve patient selection.
FOOTNOTES
Figure┬Ā1.
Radiation dose distributions of treatment plans for hepatocellular carcinoma using X-ray 3D-conformal RT (A). X-ray intensity modulated RT-volumetric modulated arc therapy (B). Proton beam therapy-passive scattering (C). Proton beam therapy-pencil beam canning (D) and the Dose-volume histogram graph of each technique (E). PBS, pencil beam scanning; IMRT, intensity modulated radiation therapy; VMAT, volumetric-modulated arc therapy.
Table┬Ā1.
Clinical outcomes of proton beam therapy in early to intermediate stage of hepatocellular carcinoma
| Author | Study design | Stage mUICC | Stage BCLC | Vascular invasion (+) | Dose/Fx | EQD2 | FFLP (3 yr) | OS (3 yr) | IHF | Toxicity (ŌēźGr3) |
|---|---|---|---|---|---|---|---|---|---|---|
| Kawashima et al. [38] (2005) (n=30) | Phase II | I (30%) | 0-B (60%) | 40% | 76 GEy/20 Fx | 87.4 Gy | 96% (2 yr) | 66% (2 yr) | 60% | 40% (acute) |
| II (63%) | C (40%) | |||||||||
| III (7%) | ||||||||||
| Chiba et al. [39] (2005) (n=162) | Retrospective | I (41%) | 6% | 50ŌĆō84 GyE/10ŌĆō24 Fx | 62.5ŌĆō94.5 Gy | 90% | 45% | 85% | 9.7% (acute) | |
| II (43%) | 3.1% (late) | |||||||||
| III (16%) | (ŌēźGrade 2) | |||||||||
| Mizumoto et al. [40] (2008) (n=53) | Retrospective | I (32%) | 28% | 72.6 GyE/22 Fx | 80.5 Gy | 86% | 45.1% | 54.7% | 0% | |
| II (30%) | ||||||||||
| III (38%) | ||||||||||
| Fukumitsu et al. [41] (2009) (n=51) | Retrospective | I (61%) | 66 GyE/10 Fx | 91.3 Gy | 94.5% | 49.2% | 56.9% | 5.9% Rib Fx | ||
| II (37%) | ||||||||||
| III (2%) | 2% RP | |||||||||
| Komatsu et al. (2011) (n=205) [27] | Retrospective | I (4%) | 0-A (38%) | 26% | 60 GyE/10 Fx | 96 Gy | 92% (5 yr) | 40% (5 yr) | 5% | |
| II-IV (96%) | B (13%) | 76 GEy/20 Fx | 105 Gy | |||||||
| C (47%) | 66 GyE/10 Fx | 109 Gy | ||||||||
| D (2%) | ||||||||||
| Nakayama et al. [42] (2011) (n=47) | Retrospective | I (43%) | 14% | 72.6ŌĆō77 | 78.3ŌĆō80.5 Gy | 88.1% | 50% | 44.7% | 2.1% GIT | |
| II (36%) | GyE/22ŌĆō31 Fx | |||||||||
| III (21%) | ||||||||||
| Kim et al. [43] (2015) (n=27) | Phase I | II (30%) | A (48%) | 60 GyE/20 Fx | 65 Gy | 71.4% | 25% | 74.1% | 0% | |
| III (55%) | B (37%) | 66 GyE/22 Fx | 71.5 Gy | 83.3% | 66.7% | |||||
| IV (15%) | C (15%) | 72 GyE/24 Fx | 78 Gy | 83.3% | 73.3% | |||||
| Hong et al. [44] (2016) (n=44) | Phase II | A/B (50%) | 29% | 58 GyE/15 Fx | 67.1 Gy | 95% (2 yr) | 63% (2 yr) | 40% (2 yr) | 2.3% | |
| C (48%) | ||||||||||
| Unknown 2% | ||||||||||
| Chadha et al. [45] (2019) (n=46) | Retrospective | A-B | 0% | 67.5 GyE/15 Fx | 97.7 Gy | 77% (2 yr) | 67% (2 yr) | 13% | ||
| Kim et al. [46] (2017) (n=71) | Retrospective | I (21%) | A (69%) | 66 GyE/10 Fx | 91.9 Gy | 89.9% | 74.4% | 69% | 0% | |
| II (72%) | B (31%) | |||||||||
| III (7%) | ||||||||||
| Kim et al. [47] (2019) (n=143) | Retrospective | I (7%) | A (56%) | 7% | 66 GyE/10 Fx | 91.9 Gy | 92.4 (5 yr) | 67.9% (5 yr) | 71.4% | 0% |
| II (42%) | B (37%) | |||||||||
| III (44%) | C (7%) | |||||||||
| IV (7%) | ||||||||||
| Kim et al. [48] (2020) (n=45) | Phase II | I (36%) | A (75.6%) | 2% | 70 GyE/10 Fx | 99.2 Gy | 95.2% | 86.4% | 73.9% | 0% |
| II (53%) | B (22.2%) | |||||||||
| III (11%) | C (2.2%) | |||||||||
| Kim et al. [49] (2021) (n=80) | Phase III | I (20%) | 0-A (60.1%) | 0% | 66 GyE/10 Fx | 91.9 Gy | 88.9% | 79.0% | PFS 20.9% | No grade |
| II (38%) | B (35.0%) | 86.5% (4 yr) | 74.0% (4 yr) | 3ŌĆō4 | ||||||
| III (41%) | C (5.0%) | |||||||||
| IV (1%) | ||||||||||
| Iwata et al. [50] (2021) (n=45) | Phase II | I (100%) | A (71%) | 0% | 66 GyE/10 Fx | 91.9 Gy | 95% (2 yr) | 84% (2 yr) | 62% (2 yr) | 2% |
| B (20%) | or 72.6 GyE/22 Fx | or 80.5 Gy | 92% (5 yr) | 70% (5 yr) | 40% (5 yr) | |||||
| C (9%) |
Dose/Fx scheme, Dose/Fractionation scheme; EQD2, equivalent dose in 2 Gy fractions, using a linear quadratic model with a/b ratios of 10 for tumor; FFLP, free from local progression; OS, overall survival; IHF, intrahepatic failure; Rib Fx, rib fracture; RP, radiation pneumonitis; GIT, gastrointestinal toxicity; BCLC, Barcelona Clinic Liver Cancer; mUICC, modified Union for International Cancer Control.
Table┬Ā2.
Clinical outcomes of proton beam therapy in advanced hepatocellular carcinoma with vascular invasion
| Author | Number | Tx modality | CPC (%) | Main VI (%) | RR (%) | 2-yr FFLP (%) | Median OS (mo) | IHF (%) | Toxicity (ŌēźGr3) |
|---|---|---|---|---|---|---|---|---|---|
| Hata et al. [59] (2005) | 12 | Proton | A (75) | 100 | 100 | 100 | 2-yr 88% | PFS | 0% |
| B (25) | 5-yr 58% | 67% (2 yr), 24% (5 yr) | |||||||
| Sugahara et al. [60] (2009) | 35 | Proton+/-TACE | A (80) | 57.1 | 82.8 | 46 | 22 | 62.9 | 5.7% leuk |
| B (20) | 2.9% throm | ||||||||
| Komatsu et al. [27] (2011) | 73 | Proton | - | - | - | 83.9% (5 yr) | 33.2% (5 yr) | - | 5% |
| Lee et al. [61] (2014) | 27 | Proton+/- Sorafenib | A (66.7) | 59.3 | 55.6 | 61.9 | 13.2 (All) | 100 | 0% |
| B (33.3) | 16 (IVA) | ||||||||
| Kim et al. [62] (2017) | 41 | Proton+/- Sorafenib | A (92.7) | 53.7 | 82.9 | 88.1 | 34.4 | 61 | 0% |
| B (7.3) | 17.2 (Main) | ||||||||
| Kim et al. [47] (2019) | 59 | Proton+/- Sorafenib | A (not reported) | 50.8 | - | - | 34.3 | - | 2.5% |
| B7 (not reported) | 19.4 (Main) | ||||||||
| Chadha et al. [45] (2019) | 13 | Proton+/- Sorafenib | - | - | - | 100% | 2-yr 44% | - | 13% |
| Su et al. [63] (2022) | 29 | Proton+ anti-PD1/PDL1 | A (100.0) | 58.6 | 51.7 | 65.1 | NR | 72.4 | 31.0% |
Abbreviations
HCC
hepatocellular carcinoma
TACE
transarterial chemoembolization
SIRT
selective internal radiotherapy
EBRT
external beam radiation therapy
IMRT
intensity modulated radiation therapy
VMAT
volumetric-modulated arc therapy
SBRT
stereotactic body radiation therapy
PBT
proton beam therapy
CIRT
carbon ion therapy
OAR
organ at risk
RILD
radiation-induced liver disease
SOBP
spread-out Bragg peak
REFERENCES
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209-249.



2. Dawson LA, Normolle D, Balter JM, McGinn CJ, Lawrence TS, Ten Haken RK. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys 2002;53:810-821.


3. Lawrence TS, Robertson JM, Anscher MS, Jirtle RL, Ensminger WD, Fajardo LF. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys 1995;31:1237-1248.


4. European Association for the Study of the Liver. EASL clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236.


5. Liang SX, Huang XB, Zhu XD, Zhang WD, Cai L, Huang HZ, et al. Dosimetric predictor identification for radiation-induced liver disease after hypofractionated conformal radiotherapy for primary liver carcinoma patients with Child-Pugh Grade A cirrhosis. Radiother Oncol 2011;98:265-269.


6. Dawson LA, Ten Haken RK. Partial volume tolerance of the liver to radiation. Semin Radiat Oncol 2005;15:279-283.


7. Kim TH, Kim DY, Park JW, Kim SH, Choi JI, Kim HB, et al. Dose-volumetric parameters predicting radiation-induced hepatic toxicity in unresectable hepatocellular carcinoma patients treated with three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys 2007;67:225-231.


8. Engelsman M, Schwarz M, Dong L. Physics controversies in proton therapy. Semin Radiat Oncol 2013;23:88-96.


9. Toramatsu C, Katoh N, Shimizu S, Nihongi H, Matsuura T, Takao S, et al. What is the appropriate size criterion for proton radiotherapy for hepatocellular carcinoma? A dosimetric comparison of spot-scanning proton therapy versus intensity-modulated radiation therapy. Radiat Oncol 2013;8:48.




10. Sun J, Wang Z, Sheng Y, Ming X, Jiang GL, Wang W. Indications of IMRT, PRT and CIRT for HCC from comparisons of dosimetry and normal tissue complication possibility. Strahlenther Onkol 2022;198:361-369.



11. Li JM, Yu JM, Liu SW, Chen Q, Mu XK, Jiang QA, et al. [Dose distributions of proton beam therapy for hepatocellular carcinoma: a comparative study of treatment planning with 3D-conformal radiation therapy or intensity-modulated radiation therapy]. Zhonghua Yi Xue Za Zhi 2009;89:3201-3206 Chinese.

12. Wang X, Krishnan S, Zhang X, Dong L, Briere T, Crane CH, et al. Proton radiotherapy for liver tumors: dosimetric advantages over photon plans. Med Dosim 2008;33:259-267.


13. Kim JY, Lim YK, Kim TH, Cho KH, Choi SH, Jeong H, et al. Normal liver sparing by proton beam therapy for hepatocellular carcinoma: Comparison with helical intensity modulated radiotherapy and volumetric modulated arc therapy. Acta Oncol 2015;54:1827-1832.


14. Smith A, Gillin M, Bues M, Zhu XR, Suzuki K, Mohan R, et al. The M. D. Anderson proton therapy system. Med Phys 2009;36:4068-4083.



15. Lomax AJ, Pedroni E, Rutz H, Goitein G. The clinical potential of intensity modulated proton therapy. Z Med Phys 2004;14:147-152.


16. Bhangoo RS, Mullikin TC, Ashman JB, Cheng TW, Golafshar MA, DeWees TA, et al. Intensity modulated proton therapy for hepatocellular carcinoma: Initial clinical experience. Adv Radiat Oncol 2021;6:100675.

17. Dionisi F, Brolese A, Siniscalchi B, Giacomelli I, Fracchiolla F, Righetto R, et al. Clinical results of active scanning proton therapy for primary liver tumors. Tumori 2021;107:71-79.


18. Yoo GS, Yu JI, Cho S, Jung SH, Han Y, Park S, et al. Comparison of clinical outcomes between passive scattering versus pencil-beam scanning proton beam therapy for hepatocellular carcinoma. Radiother Oncol 2020;146:187-193.


19. Simone CB 2nd, Plastaras JP, Jabbour SK, Lee A, Lee NY, Choi JI, et al. Proton reirradiation: Expert recommendations for reducing toxicities and offering new chances of cure in patients with challenging recurrence malignancies. Semin Radiat Oncol 2020;30:253-261.


20. Hashimoto T, Tokuuye K, Fukumitsu N, Igaki H, Hata M, Kagei K, et al. Repeated proton beam therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2006;65:196-202.


21. McDuff SGR, Remillard KA, Zheng H, Raldow AC, Wo JY, Eyler CE, et al. Liver reirradiation for patients with hepatocellular carcinoma and liver metastasis. Pract Radiat Oncol 2018;8:414-421.


22. Oshiro Y, Mizumoto M, Okumura T, Fukuda K, Fukumitsu N, Abei M, et al. Analysis of repeated proton beam therapy for patients with hepatocellular carcinoma. Radiother Oncol 2017;123:240-245.


23. Cheng JY, Liu CM, Wang YM, Hsu HC, Huang EY, Huang TT, et al. Proton versus photon radiotherapy for primary hepatocellular carcinoma: a propensity-matched analysis. Radiat Oncol 2020;15:159.


24. Qi WX, Fu S, Zhang Q, Guo XM. Charged particle therapy versus photon therapy for patients with hepatocellular carcinoma: a systematic review and meta-analysis. Radiother Oncol 2015;114:289-295.


25. Hasan S, Abel S, Verma V, Webster P, Arscott WT, Wegner RE, et al. Proton beam therapy versus stereotactic body radiotherapy for hepatocellular carcinoma: practice patterns, outcomes, and the effect of biologically effective dose escalation. J Gastrointest Oncol 2019;10:999-1009.



26. Suzuki M, Kase Y, Yamaguchi H, Kanai T, Ando K. Relative biological effectiveness for cell-killing effect on various human cell lines irradiated with heavy-ion medical accelerator in Chiba (HIMAC) carbon-ion beams. Int J Radiat Oncol Biol Phys 2000;48:241-250.


27. Komatsu S, Fukumoto T, Demizu Y, Miyawaki D, Terashima K, Sasaki R, et al. Clinical results and risk factors of proton and carbon ion therapy for hepatocellular carcinoma. Cancer 2011;117:4890-4904.


28. Kato H, Tsujii H, Miyamoto T, Mizoe JE, Kamada T, Tsuji H, et al. Results of the first prospective study of carbon ion radiotherapy for hepatocellular carcinoma with liver cirrhosis. Int J Radiat Oncol Biol Phys 2004;59:1468-1476.


29. Gandhi SJ, Liang X, Ding X, Zhu TC, Ben-Josef E, Plastaras JP, et al. Clinical decision tool for optimal delivery of liver stereotactic body radiation therapy: Photons versus protons. Pract Radiat Oncol 2015;5:209-218.


30. Arscott WT, Thompson RF, Yin L, Burgdorf B, Kirk M, Ben-Josef E. Stereotactic body proton therapy for liver tumors: Dosimetric advantages and their radiobiological and clinical implications. Phys Imaging Radiat Oncol 2018;8:17-22.



31. Chuong MD, Kaiser A, Khan F, Parikh P, Ben-Josef E, Crane C, et al. Consensus report from the miami liver proton therapy conference. Front Oncol 2019;9:457.



32. Lasley FD, Mannina EM, Johnson CS, Perkins SM, Althouse S, Maluccio M, et al. Treatment variables related to liver toxicity in patients with hepatocellular carcinoma, Child-Pugh class A and B enrolled in a phase 1-2 trial of stereotactic body radiation therapy. Pract Radiat Oncol 2015;5:e443-e449.


33. Hata M, Tokuuye K, Sugahara S, Fukumitsu N, Hashimoto T, Ohnishi K, et al. Proton beam therapy for hepatocellular carcinoma patients with severe cirrhosis. Strahlenther Onkol 2006;182:713-720.

34. Llovet JM, Br├║ C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329-338.


36. Kwak HW, Park JW, Nam BH, Yu A, Woo SM, Kim TH, et al. Clinical outcomes of a cohort series of patients with hepatocellular carcinoma in a hepatitis B virus-endemic area. J Gastroenterol Hepatol 2014;29:820-829.

37. Korean Liver Cancer Study Group and National Cancer Center, Korea. [Practice guidelines for management of hepatocellular carcinoma 2009]. Korean J Hepatol 2009;15:391-423 Korean.


38. Kawashima M, Furuse J, Nishio T, Konishi M, Ishii H, Kinoshita T, et al. Phase II study of radiotherapy employing proton beam for hepatocellular carcinoma. J Clin Oncol 2005;23:1839-1846.


39. Chiba T, Tokuuye K, Matsuzaki Y, Sugahara S, Chuganji Y, Kagei K, et al. Proton beam therapy for hepatocellular carcinoma: a retrospective review of 162 patients. Clin Cancer Res 2005;11:3799-3805.



40. Mizumoto M, Tokuuye K, Sugahara S, Nakayama H, Fukumitsu N, Ohara K, et al. Proton beam therapy for hepatocellular carcinoma adjacent to the porta hepatis. Int J Radiat Oncol Biol Phys 2008;71:462-467.


41. Fukumitsu N, Sugahara S, Nakayama H, Fukuda K, Mizumoto M, Abei M, et al. A prospective study of hypofractionated proton beam therapy for patients with hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2009;74:831-836.


42. Nakayama H, Sugahara S, Fukuda K, Abei M, Shoda J, Sakurai H, et al. Proton beam therapy for hepatocellular carcinoma located adjacent to the alimentary tract. Int J Radiat Oncol Biol Phys 2011;80:992-995.

43. Kim TH, Park JW, Kim YJ, Kim BH, Woo SM, Moon SH, et al. Phase I dose-escalation study of proton beam therapy for inoperable hepatocellular carcinoma. Cancer Res Treat 2015;47:34-45.




44. Hong TS, Wo JY, Yeap BY, Ben-Josef E, McDonnell EI, Blaszkowsky LS, et al. Multi-institutional phase II study of high-dose hypofractionated proton beam therapy in patients with localized, Unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol 2016;34:460-468.



45. Chadha AS, Gunther JR, Hsieh CE, Aliru M, Mahadevan LS, Venkatesulu BP, et al. Proton beam therapy outcomes for localized unresectable hepatocellular carcinoma. Radiother Oncol 2019;133:54-61.



46. Kim TH, Park JW, Kim BH, Kim DY, Moon SH, Kim SS, et al. Optimal time of tumour response evaluation and effectiveness of hypofractionated proton beam therapy for inoperable or recurrent hepatocellular carcinoma. Oncotarget 2017;9:4034-4043.



47. Kim TH, Park JW, Kim BH, Kim H, Moon SH, Kim SS, et al. Does risk-adapted proton beam therapy have a role as a complementary or alternative therapeutic option for hepatocellular carcinoma? Cancers (Basel) 2019;11:230.



48. Kim TH, Park JW, Kim BH, Oh ES, Youn SH, Moon SH, et al. Phase II study of hypofractionated proton beam therapy for hepatocellular carcinoma. Front Oncol 2020;10:542.



49. Kim TH, Koh YH, Kim BH, Kim MJ, Lee JH, Park B, et al. Proton beam radiotherapy vs. radiofrequency ablation for recurrent hepatocellular carcinoma: A randomized phase III trial. J Hepatol 2021;74:603-612.


50. Iwata H, Ogino H, Hattori Y, Nakajima K, Nomura K, Hashimoto S, et al. A Phase 2 study of image-guided proton therapy for operable or ablation-treatable primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2021;111:117-126.


51. Terayama N, Miyayama S, Tatsu H, Yamamoto T, Toya D, Tanaka N, et al. Subsegmental transcatheter arterial embolization for hepatocellular carcinoma in the caudate lobe. J Vasc Interv Radiol 1998;9:501-508.

52. Bush DA, Volk M, Smith JC, Reeves ME, Sanghvi S, Slater JD, et al. Proton beam radiotherapy versus transarterial chemoembolization for hepatocellular carcinoma: Results of a randomized clinical trial. Cancer. 2023 Jul 28. doi: 10.1002/cncr.34965.


53. Fukuda K, Okumura T, Abei M, Fukumitsu N, Ishige K, Mizumoto M, et al. Long-term outcomes of proton beam therapy in patients with previously untreated hepatocellular carcinoma. Cancer Sci 2017;108:497-503.




54. Kim TH, Kim BH, Park JW, Cho YR, Koh YH, Chun JW, et al. Proton beam therapy for treatment-naïve hepatocellular carcinoma and prognostic significance of albumin-bilirubin (ALBI) grade. Cancers (Basel) 2022;14:4445.



55. Korean Liver Cancer Association (KLCA) and National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol 2022;28:583-705.




56. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382:1894-1905.


57. Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH, An JH, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: A randomized clinical trial. JAMA Oncol 2018;4:661-669.



58. Wei X, Jiang Y, Zhang X, Feng S, Zhou B, Ye X, et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: A randomized, open-label, multicenter controlled study. J Clin Oncol 2019;37:2141-2151.



59. Hata M, Tokuuye K, Sugahara S, Kagei K, Igaki H, Hashimoto T, et al. Proton beam therapy for hepatocellular carcinoma with portal vein tumor thrombus. Cancer 2005;104:794-801.


60. Sugahara S, Nakayama H, Fukuda K, Mizumoto M, Tokita M, Abei M, et al. Proton-beam therapy for hepatocellular carcinoma associated with portal vein tumor thrombosis. Strahlenther Onkol 2009;185:782-788.



61. Lee SU, Park JW, Kim TH, Kim YJ, Woo SM, Koh YH, et al. Effectiveness and safety of proton beam therapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Strahlenther Onkol 2014;190:806-814.



62. Kim DY, Park JW, Kim TH, Kim BH, Moon SH, Kim SS, et al. Risk-adapted simultaneous integrated boost-proton beam therapy (SIB-PBT) for advanced hepatocellular carcinoma with tumour vascular thrombosis. Radiother Oncol 2017;122:122-129.


-
METRICS

- ORCID iDs
-
Tae Hyun Kim

https://orcid.org/0000-0001-8413-3385 - Related articles
-
Recent advances in the management of hepatocellular carcinoma2024 January;30(1)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



