| Clin Mol Hepatol > Volume 30(3); 2024 > Article |
|
ABSTRACT
Background/Aims
Methods
Results
ACKNOWLEDGMENTS
FOOTNOTES
SUPPLEMENTAL MATERIAL
Supplementary Figure 1.
Supplementary Figure 2.
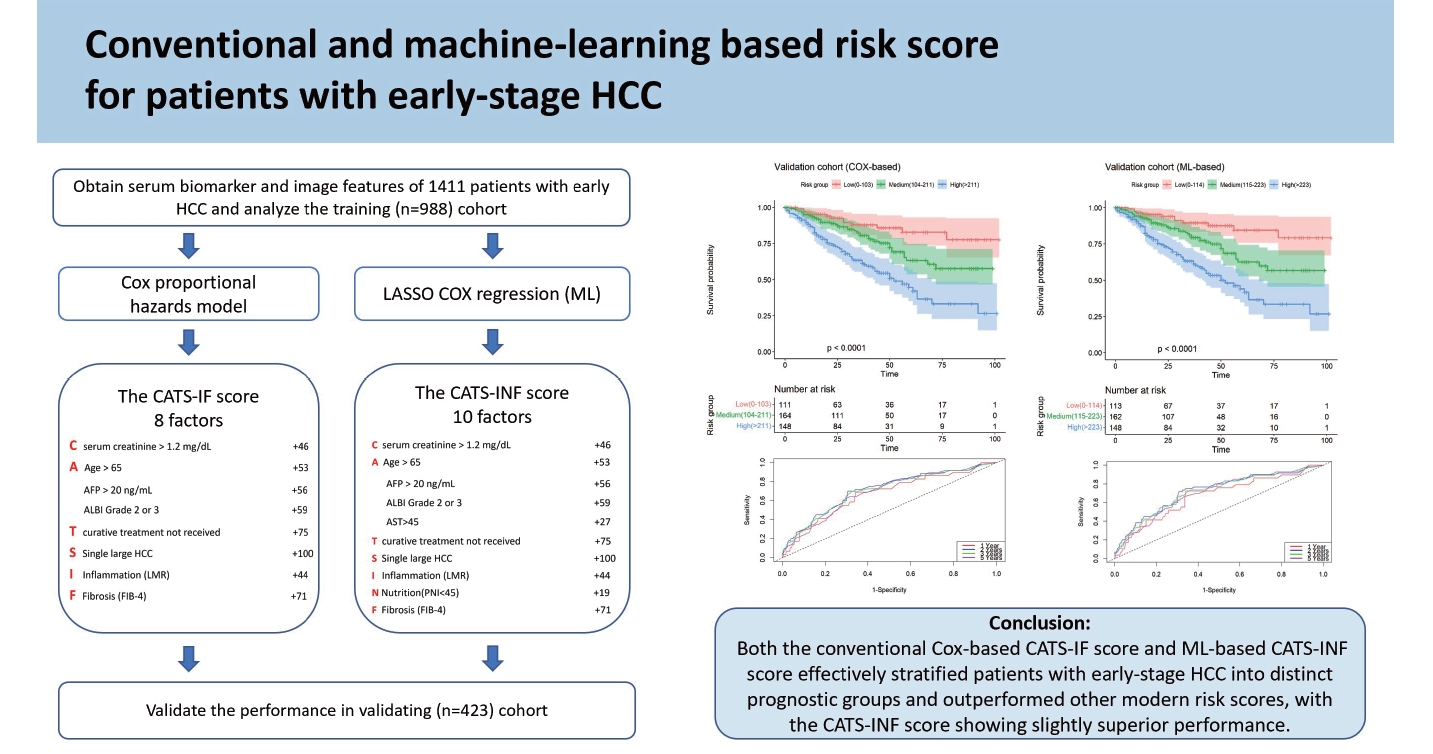
Figure 1.
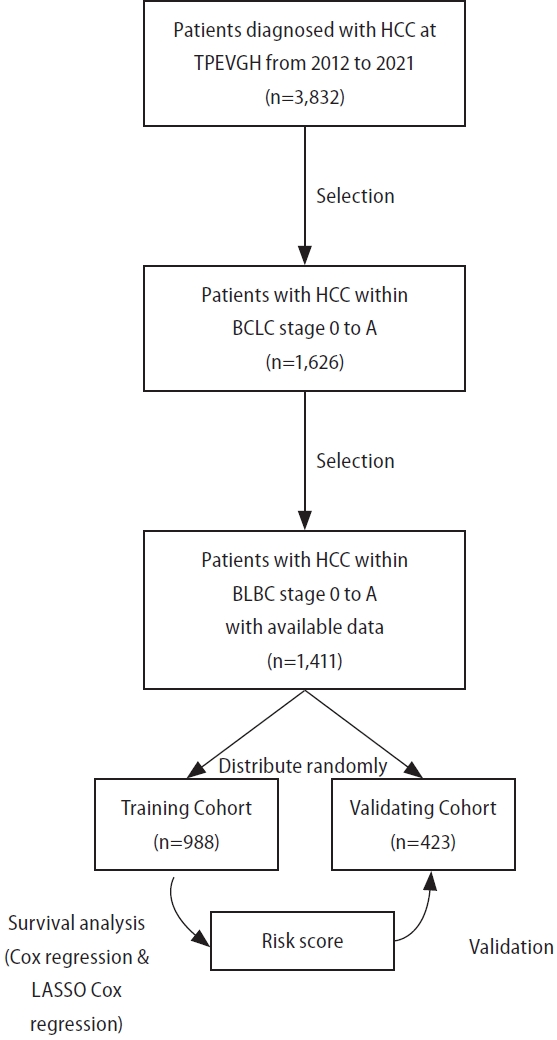
Figure 2.
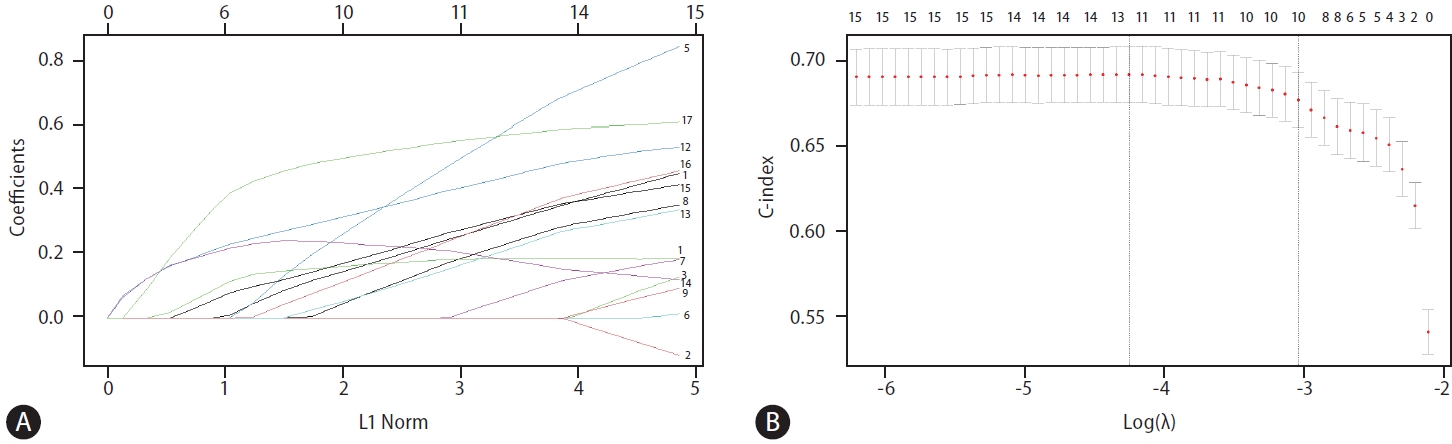
Figure 3.
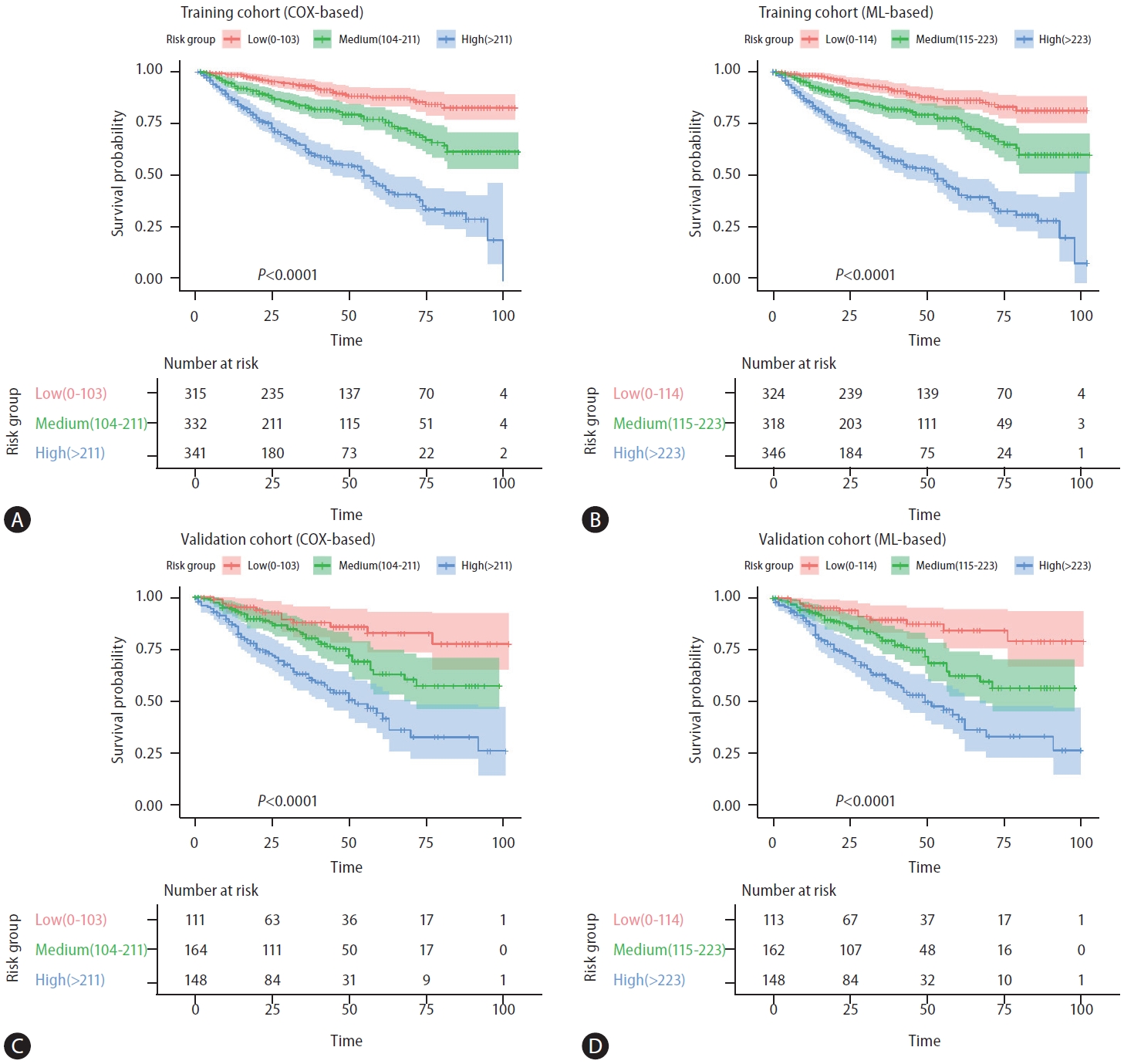
Figure 4.
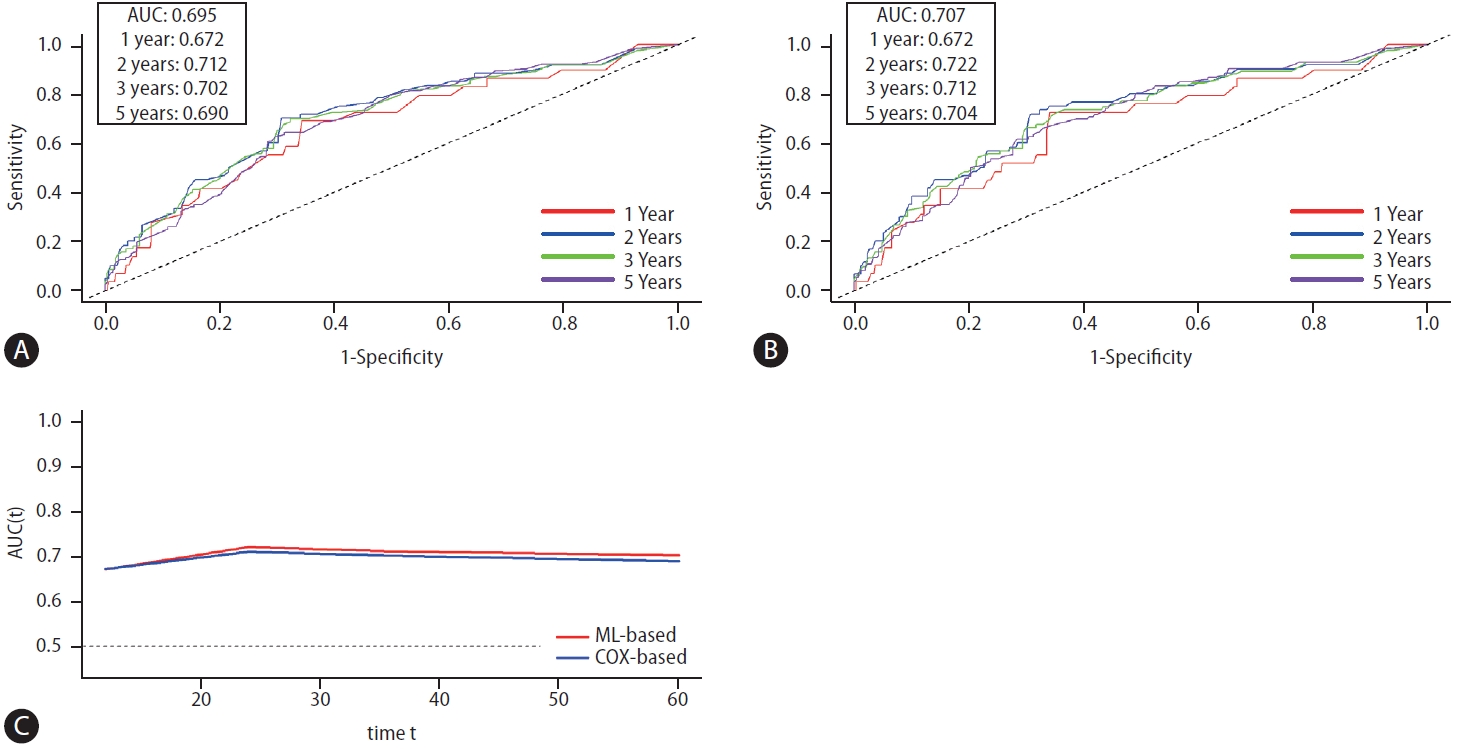
Table 1.
Continuous variables are expressed as the median with the 25th and 75th percentiles.
HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; SLHCC, single large hepatocellular carcinoma; ALT, alanine aminotransferase; AST, aspartate aminotransferase; LMR, lymphocyte-to-monocyte ratio; PNI, prognostic nutritional index; FIB-4, fibrosis-4 index; ALBI, albumin–bilirubin; AFP, alpha fetoprotein.
Table 2.
HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; SLHCC, single large hepatocellular carcinoma; ALT, alanine aminotransferase; AST, aspartate aminotransferase; LMR, lymphocyte-to-monocyte ratio; PNI, prognostic nutritional index; FIB-4, fibrosis-4 index; ALBI, albumin–bilirubin; AFP, alpha fetoprotein; HR, hazard ratio, CI, confidence interval.
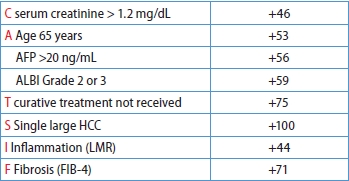
Table 3.
SLHCC, single large hepatocellular carcinoma; FIB-4, fibrosis-4 index; LMR, lymphocyte-to-monocyte ratio; PNI, prognostic nutritional index; ALBI, albumin–bilirubin; AFP, alpha fetoprotein.
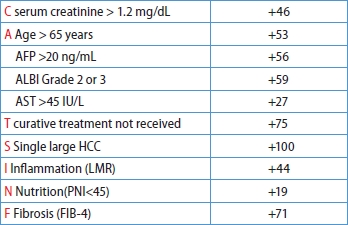
Table 3.
SLHCC, single large hepatocellular carcinoma; FIB-4, fibrosis-4 index; LMR, lymphocyte-to-monocyte ratio; PNI, prognostic nutritional index; ALBI, albumin–bilirubin; AFP, alpha fetoprotein.
Table 4.
Abbreviations
REFERENCES
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,132 View
- 104 Download
- ORCID iDs
-
Elise Chia-Hui Tan

https://orcid.org/0000-0002-8115-3011Chien-Wei Su

https://orcid.org/0000-0003-3889-7004 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement1
Supplement1 Print
Print



