| Clin Mol Hepatol > Volume 29(2); 2023 > Article |
|
ABSTRACT
Background/Aims
Methods
Results
ACKNOWLEDGMENTS
FOOTNOTES
Supplementary materials
Supplementary Table 1.
Supplementary Table 2.
Supplementary Table 3.
Supplementary Figure 1.
Figure 1.
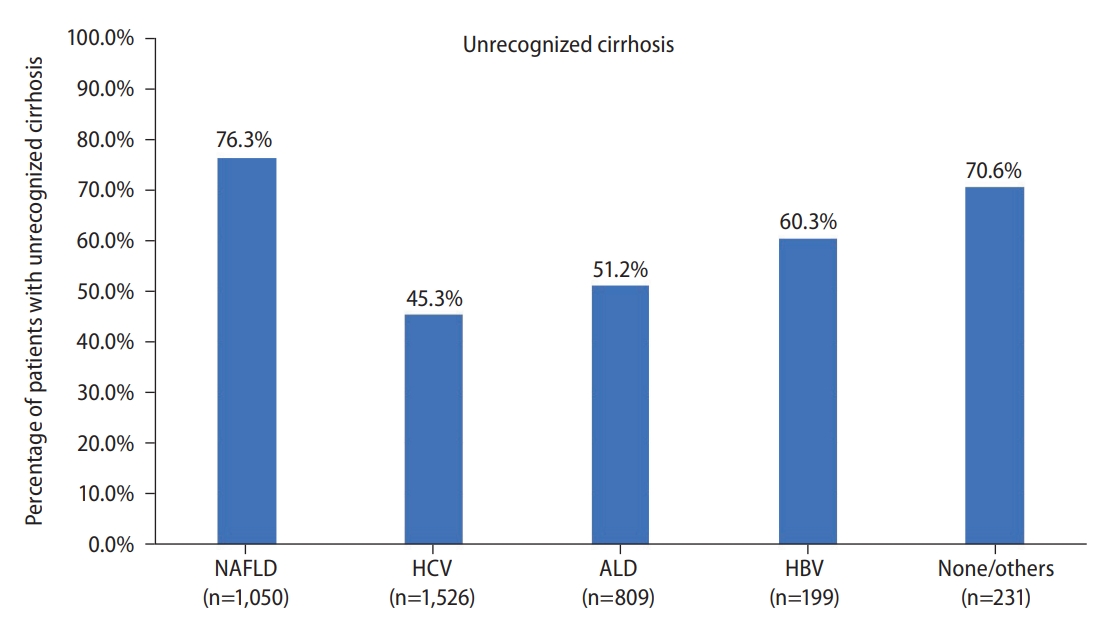
Figure 2.
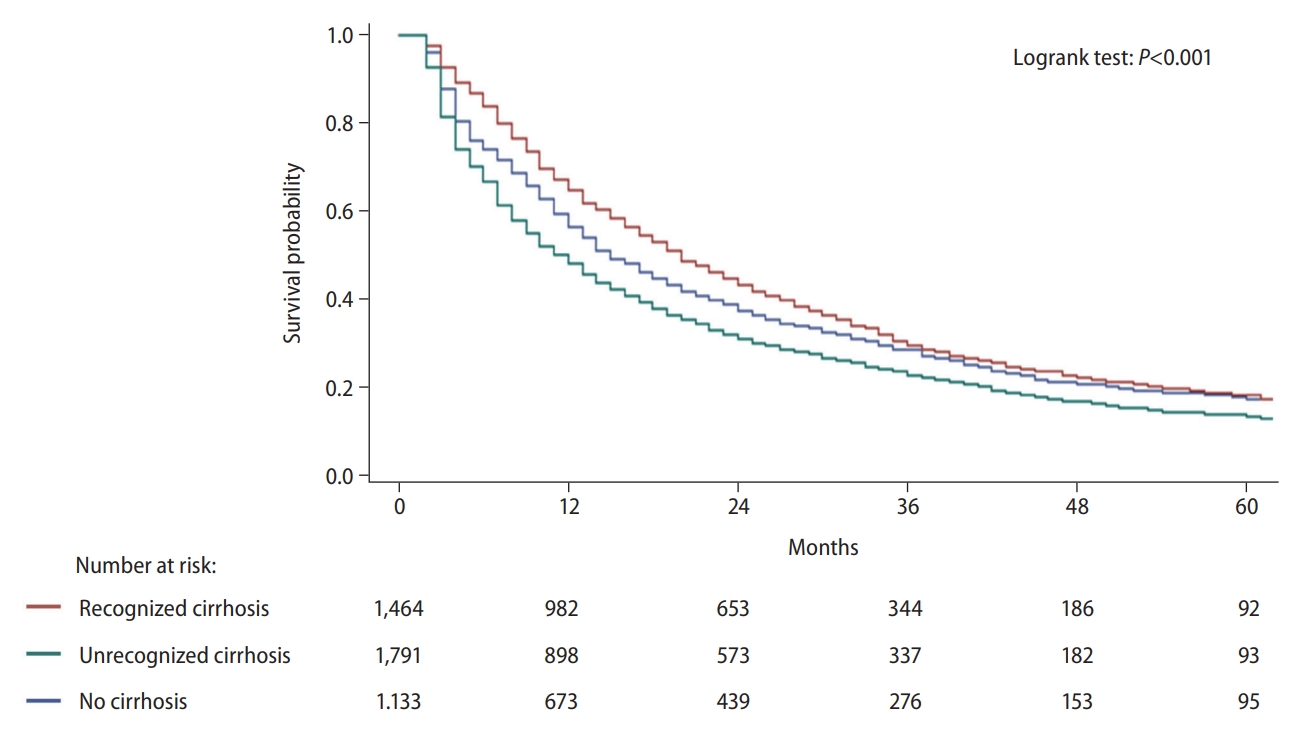
Table 1.
| Characteristics | Recognized cirrhosis (n=1,625) | Unrecognized cirrhosis (n=2,190) | No cirrhosis (n=1,283) | P-value | |
|---|---|---|---|---|---|
| Male Sex | 962 (59.2) | 1,569 (71.6) | 907 (70.7) | <0.001 | |
| Age | 75.2±5.67 | 76.8±6.11 | 78.6±6.54 | <0.001 | |
| Race/ethnicity | <0.001 | ||||
| Non-Hispanic White | 968 (59.6) | 1,378 (63.0) | 878 (68.4) | - | |
| Non-Hispanic Black | 100 (6.1) | 180 (8.2) | 110 (8.6) | - | |
| Non-Hispanic API/Others | 257 (15.8) | 360 (16.4) | 193 (15.0) | - | |
| Hispanic | 300 (18.5) | 272 (12.4) | 102 (8.0) | - | |
| Poverty level | 0.76 | ||||
| 0% to <5% poverty | 318 (19.6) | 447 (20.4) | 246 (19.2) | - | |
| 5% to <10% poverty | 363 (22.3) | 520 (23.7) | 309 (24.1) | - | |
| 10% to <20% poverty | 517 (31.8) | 659 (30.1) | 384 (29.9) | - | |
| 20% to 100% poverty | 427 (26.3) | 564 (25.8) | 344 (26.8) | - | |
| Rural-Urban | 0.002 | ||||
| Metro >1 million | 986 (60.7) | 1,333 (60.9) | 710 (55.3) | - | |
| Metro 250k to1 million | 340 (20.9) | 408 (18.6) | 280 (21.8) | - | |
| Metro <250k | 132 (8.1) | 162 (7.4) | 120 (9.4) | - | |
| Non-Metro/Rural | 167 (10.3) | 287 (13.1) | 173 (13.5) | - | |
| NCI Comorbidity Index | 0.67 | ||||
| Low (0 to 2) | 1,223 (75.3) | 1,660 (75.8) | 959 (74.7) | - | |
| Moderate (>2 to 4) | 221 (13.6) | 312 (14.2) | 192 (15.0) | - | |
| High (>4) | 181 (11.1) | 218 (10.0) | 132 (10.3) | - | |
| Etiology | <0.001 | ||||
| HCV | 834 (51.3) | 692 (31.6) | 189 (14.7) | - | |
| NAFLD | 249 (15.3) | 801 (36.6) | 763 (59.5) | - | |
| ALD | 395 (24.3) | 414 (18.9) | 86 (6.7) | - | |
| HBV | 79 (4.9) | 120 (5.5) | 47 (3.7) | - | |
| Other/None | 68 (4.2) | 163 (7.4) | 198 (15.4) | - | |
| Diabetes | 1,083 (66.6) | 1,408 (64.3) | 771 (60.1) | <0.001 | |
| Ascites | 986 (60.7) | 1,227 (56.0) | 0 (0) | <0.001 | |
| Hepatic encephalopathy | 540 (33.2) | 335 (15.3) | 0 (0) | <0.001 | |
| Early stage* | 485 (29.9) | 301 (13.7) | 181 (14.1) | <0.001 | |
| Curative treatment† | 474 (29.2) | 435 (19.9) | 258 (20.1) | <0.001 | |
Values are presented as number (%) or mean±standard deviation.
ALD, alcoholic liver disease; API, Asian/Pacific Islander; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; Metro, metropolitan; NAFLD, nonalcoholic fatty liver disease; NCI, National Cancer Institute.
Table 2.
Table 3.
Table 4.
| Characteristics |
Univariate analysis |
Multivariable analysis |
|||
|---|---|---|---|---|---|
| HR (95% CI) | P-value | aHR (95% CI) | P-value | ||
| Cirrhosis status | |||||
| Recognized cirrhosis | Ref | Ref | Ref | Ref | |
| Unrecognized cirrhosis | 1.35 (1.25 to 1.46) | <0.001 | 1.17 (1.08 to 1.27) | <0.001 | |
| No cirrhosis | 1.11 (1.02 to 1.21) | 0.02 | 0.84 (0.76 to 0.93) | 0. 001 | |
| Male sex (ref. female) | 1.16 (1.08 to 1.25) | <0.001 | 1.06 (0.98 to 1.14) | 0.14 | |
| Age | 1.03 (1.02 to 1.03) | <0.001 | 1.01 (1.00 to 1.02) | <0.001 | |
| Race/ethnicity | |||||
| Non-Hispanic White | Ref | Ref | Ref | Ref | |
| Non-Hispanic Black | 1.01 (0.89 to 1.15) | 0.86 | 0.97 (0.85 to 1.12) | 0.70 | |
| Non-Hispanic API/Others | 0.65 (0.59 to 0.72) | <0.001 | 0.77 (0.69 to 0.86) | <0.001 | |
| Hispanic | 0.94 (0.85 to 1.04) | 0.22 | 0.92 (0.83 to 1.02) | 0.12 | |
| Census Poverty Level | |||||
| <5% | Ref | Ref | Ref | Ref | |
| 5% to <10% | 1.14 (1.03 to 1.26) | 0.01 | 1.06 (0.95 to 1.17) | 0.30 | |
| 10% to <20% | 1.12 (1.02 to 1.24) | 0.02 | 1.03 (0.93 to 1.14) | 0.55 | |
| 20% to 100% | 1.14 (1.03 to 1.26) | 0.009 | 1.08 (0.97 to 1.20) | 0.17 | |
| Rural-Urban | |||||
| Metro >1 million | Ref | Ref | Ref | Ref | |
| Metro 250k to 1 million | 1.15 (1.06 to 1.25) | 0.001 | 1.14 (1.05 to 1.24) | 0.002 | |
| Metro <250k | 1.23 (1.09 to 1.39) | 0.001 | 1.07 (0.94 to 1.21) | 0.29 | |
| Non-Metro/Rural | 1.28 (1.15 to 1.42) | <0.001 | 1.12 (1.00 to 1.25) | 0.04 | |
| NCI comorbidity index | |||||
| Low (0 to 2) | Ref | Ref | Ref | Ref | |
| Moderate (>2 to 4) | 1.23 (1.11 to 1.35) | <0.001 | 1.09 (0.99 to 1.20) | 0.09 | |
| High (>4) | 1.60 (1.43 to 1.78) | <0.001 | 1.35 (1.21 to 1.51) | <0.001 | |
| Etiology | |||||
| HCV | Ref | Ref | Ref | Ref | |
| NAFLD | 1.37 (1.27 to 1.49) | <0.001 | 1.19 (1.09 to 1.31) | <0.001 | |
| ALD | 1.36 (1.24 to 1.50) | <0.001 | 1.18 (1.06 to 1.30) | 0.001 | |
| HBV | 0.87 (0.73 to 1.03) | 0.10 | 1.08 (0.91 to 1.28) | 0.40 | |
| Other/None | 1.21 (1.06 to 1.38) | 0.004 | 1.13 (0.98 to 1.29) | 0.09 | |
| Diabetes | 1.09 (1.02 to 1.17) | 0.01 | 1.04 (0.97 to 1.12) | 0.29 | |
| Early stage* | 0.46 (0.42 to 0.50) | <0.001 | 0.54 (0.50 to 0.60) | <0.001 | |
| Curative treatment† | 0.27 (0.25 to 0.30) | <0.001 | 0.30 (0.28 to 0.33) | <0.001 | |
aHR, adjusted hazard ratio; ALD, alcoholic liver disease; API, Asian/Pacific Islander; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HR, hazard ratio; Metro, metropolitan; NAFLD, nonalcoholic fatty liver disease; NCI, National Cancer Institute.
Abbreviations
REFERENCES
- TOOLS
-
METRICS

- ORCID iDs
-
Ju Dong Yang

https://orcid.org/0000-0001-7834-9825 - Related articles
-
Carbon Ion Radiotherapy in the Treatment of Hepatocellular Carcinoma2023 October;29(4)
Recent advances in the management of hepatocellular carcinoma2024 January;30(1)
Systemic therapy in advanced hepatocellular carcinoma2023 April;29(2)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement1
Supplement1 Print
Print



