| Clin Mol Hepatol > Volume 29(4); 2023 > Article |
|
ABSTRACT
FOOTNOTES
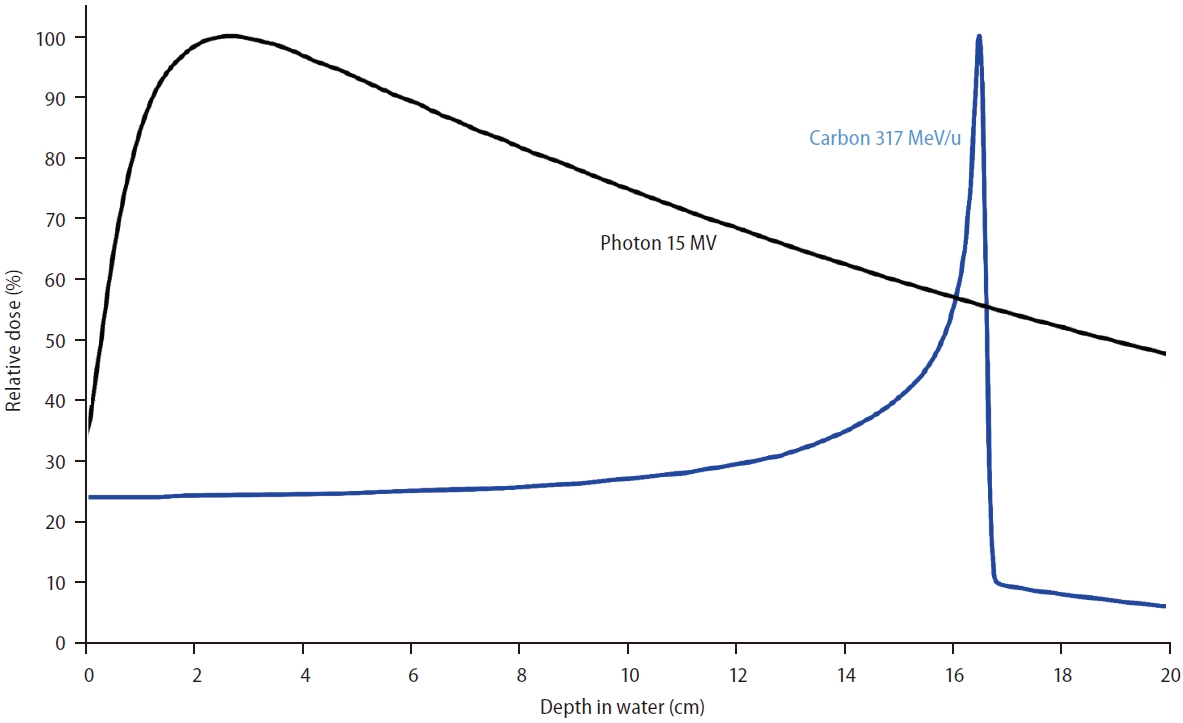
Figure 2.

Table 1.
| Author, yr, center | No. of pts | Total dose, Gy (RBE)/fractions | Median follow-up, months (range) | Tumor size | No. of tumors | Macrovascular invasion | Liver function (Child-Pugh class) | Local control | Progression-free survival | Overall survival | Grade 3+ Toxicities |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Prospective phase 1/2 trials | |||||||||||
| Kato et al., 2004, NIRS [21] | 24 | 49.5–79.5 Gy/15 fr | 71 (63–83) | Median 5 cm | One: 21 pts | NR | A: 16 pts | 1 yr: 92% | NR | 1 yr: 92% | Acute: gr 3 skin toxicity, 1 pt; gr 3 leucopenia and thrombocytopenia, 5 pts |
| Two: 3 pts | B: 8 pts | 3 yr: 81% | 3 yr: 50% | ||||||||
| 5 yr: 81% | 5 yr: 25% | Late: gr 3 thrombocytopenia, 3 pts (one died of variceal bleed and hepatic failure) | |||||||||
| Kasuya et al., 2017, NIRS [20] | 126 | 69.6 Gy/12 fr, 58 Gy/8 fr, 52.8 Gy/4 fr | 27.1 (0.9–154.8) | Median 4 cm | Single: 103 tumors | Present in 23 or 133 tumors | A: 97 pts | 1 yr: 94.7% | NR | 1 yr: 90.3% | Acute: gr 3 skin toxicity, 3 pts |
| Multiple: 30 tumors | B: 29 pts | 3 yr: 91.4% | 3 yr: 50.0% | ||||||||
| 5 yr: 90.0% | 5 yr: 25.0% | Late: gr 3 skin, 3 pts; gr 3 pleural effusion, 1 pt | |||||||||
| Shibuya et al., 2019, Gunma [28] | 21 | 60 Gy/4 fr | 24.2 (6.3–43.7) | Median 4.8 cm | Single: 20 pts | None | A: 21 pts | 1 yr: 100% | 1 yr: 81.0% | 1 yr: 90.5% | Acute: none |
| Multiple: 1 pt | 2 yr: 92.3% | 2 yr: 50.0% | 2 yr: 80.0% | Late: gr 3 cholecystitis and encephalopathy, 2 pts; gr 3 other toxicity (not specified), 1 pt | |||||||
| Shibuya et al., 2021, Gunma [27] | 35 | 52.8 Gy/4 fr, 60 Gy/4 fr | 49.0 (4.0–62.4) | Median 3.5 cm | Single: 34 pts | None | A: 29 pts | 2 yr: 92.6% | 2 yr: 45.7% | 2 yr: 82.8% | Acute: none |
| Multiple: 1 pt | B: 6 pts | 3 yr: 76.5% | 3 yr: 33.8% | 3 yr: 76.7% | Late: gr 3 hepatobiliary toxicity, 2 pts | ||||||
| 4 yr: 76.5% | 4 yr: 29.5% | 4 yr: 69.4% | |||||||||
| Hong et al., 2023, SPHIC [17] | 23 | 55–70 Gy/10 fr | 56.1 (5.7–74.4) | Median 4.3 cm | Single: 15 pts | Present in 6 pts | A: 23 pts | 1 yr: 100% | 1 yr: 73.6% | 1 yr: 91.3% | Acute: gr 3 leukocytopenia, 2 pts |
| Multiple: 8 pts | 3 yr: 94.4% | 3 yr: 59.2% | 3 yr: 81.9% | ||||||||
| 5 yr: 94.4% | 5 yr: 37.0% | 5 yr: 67.1% | Late: gr 3 stomach bleeding, 2 pts | ||||||||
| Retrospective studies | |||||||||||
| Imada et al., 2010, NIRS [19] | 64 | 52.8 Gy/4 fr | Porta hepatis: 34 (6–90) | Porta hepatis group: median 3.7 cm | Porta hepatis group: | Porta hepatis group: present in 16 pts | Porta hepatis group | Porta hepatis group | Porta hepatis group | Porta hepatis group | Acute: gr 3 liver toxicity, 3 pts, gr 3 hematologic toxicity, 6 pts (porta hepatis) gr 3 liver toxicity, 8 pts, gr 3 hematologic toxicity, 8 pts (nonporta hepatis) |
| Single: 15 pts | A: 16 pts | 3 yr: 87.8% | 3 yr: 5.6% | 3 yr: 44.4% | |||||||
| Multiple: 3 pts | B: 2 pts | 5 yr: 87.8% | 3 yr: 5.6% | 5 yr: 22.2% | |||||||
| Non-porta hepatis: 41 (11–98) | Non-porta hepatis group: median 4 cm | Non-porta hepatis group: | Non-porta hepatis group: present in 29 pts | Non-porta hepatis group | Non-porta hepatis group | Non-porta hepatis | Non-porta hepatis group | ||||
| Single: 41 pts | A: 33 pts | 3 yr: 95.7% | 3 yr: 34.8% | 3 yr: 60.9% | |||||||
| Multiple: 5 pts | B: 13 pts | 5 yr: 95.7% | 5 yr: 23.9% | 5 yr: 34.8% | Late: NR | ||||||
| Imada et al., 2010, NIRS [18] | 43 | 48.0–79.5 Gy/4–15 fr | NR | NR | Compensatory enlargement of liver | NR | Compensatory enlargement of liver | NR | Compensatory enlargement of liver 3 yr: | Compensatory enlargement of liver | NR |
| Larger group | Larger group | Larger group | Larger group | ||||||||
| One: 19 pts | A: 18 pts | 3 yr: 50.0% | 3 yr: 80.0% | ||||||||
| Two: 1 pt | B: 2 pts | 5 yr: 28.0% | 5 yr: 48.9% | ||||||||
| Smaller group | Smaller group | Smaller group | Smaller group | ||||||||
| One: 17 pts | A: 17 pts | 3 yr: 26.1% | 3 yr: 52.2% | ||||||||
| Two: 6 pts | B: 6 pts | 5 yr: 0.0% | 5 yr: 29.4% | ||||||||
| Komatsu et al., 2011, HIBMC [22] | 101 | 52.8 Gy/4 fr, 52.8 Gy/8 fr, 66 Gy/10 fr, 76 Gy/20 fr | 31 | <5 cm: 81 tumors | Single: 81 pts | Present in 19 of 108 tumors | A: 78 pts | 5 yr: 93% | NR | 5 yr: 36.3% | Acute: none |
| 2–10 cm: 22 tumors | Multiple: 20 pts | B: 20 pts | Late: gr 3 elevation of transaminase level, 3 pts; gr 3 subcutaneous panniculitis, 1 pt | ||||||||
| >10 cm: 5 tumors | C: 3 pts | ||||||||||
| Habermehl et al., 2013, HIT [15] | 6 | 40 Gy/4 fr | 11 (3.4–12.7) | Median 3.5 cm | One: 3 pts | NR | A: 4 pts | NR | NR | NR | Acute: none |
| Two: 2 pts | B: 1 pt | Late: none | |||||||||
| Multiple: 1 pt | |||||||||||
| Shiba et al., 2017, Gunma [23] | 31 | 52.8 Gy/4 fr, 60.0 Gy/4 fr, 60.0 Gy/12 fr | 23.2 (8.4–55.3) | Median 4.5 cm | Single: 31 pts | Present in 6 pts | A: 27 pts | 2 yr: 89.2% | 2 yr: 51.3% | 2 yr: 82.3% | Acute: none |
| B: 4 pts | Late: gr 3 encephalopathy, 3 pts | ||||||||||
| Shibuya et al., 2018, J-CROS [13] | 174 | 48 Gy/2 fr, 52.8/4 fr, 60/4 fr | 20.3 (2.9–103.5) | Median 3.0 cm | One: 157 pts | None | A: 153 pts | 1 yr: 94.6% | NR | 1 yr: 95.4% | Acute: gr 3 dermatitis, 2 pts; gr 3 elevation of AST, 1 pt |
| Two: 15 pts | B: 20 pts | 3 yr: 81.0% | 3 yr: 73.3% | Late: gr 3 dermatitis, 4 pts; gr 3 myopathy, 1 pt; gr 3 rib fracture, 1 pt; gr 4 dermatitis, 1 pt | |||||||
| Three: 2 pts | |||||||||||
| Shiba et al., 2018, Gunma [25] | 68 | 52.8 Gy/4 fr, 60.0 Gy/4 fr | 33.5 (3.9–83.1) | Sarcopenia: 3 cm | NR | NR | Sarcopenia: | 3 yr: | 3 yr: | 3 yr: | Acute: none |
| Non-sarcopenia: 3.6 cm | A: 17 pts | Sarcopenia: 81% | Sarcopenia: 46% | Sarcopenia: 66% | Late: gr 3 encephalopathy, 2 pts | ||||||
| B: 5 pts | |||||||||||
| Non-sarcopenia: | Non- sarcopenia: 72% | Non-sarcopenia: 30% | Non-sarcopenia: 77% | ||||||||
| A: 40 pts | |||||||||||
| B: 6 pts | |||||||||||
| Shiba et al., 2019, Gunma [24] | 31 | 52.8 Gy/4 fr, 60.0 Gy/4 fr, 60.0 Gy/12 fr | 43 (4–84) | Median 3.4 cm | Single: 31 pts | None | A: 29 pts | 3 yr: 80% | 3 yr: 51% | 3 yr: 88% | NR |
| B: 2 pts | |||||||||||
| Shiba et al., 2020, Gunma [26] | 11 | 52.8 Gy/4 fr, 60.0 Gy/4 fr, 60.0 Gy/12 fr | 36.4 (4.3–86.2) | Median 5.3 cm | NR | Present in 11 pts | A: 10 pts | 3 yr: 78% | 3 yr: 18% | 3 yr: 64% | Acute: none |
| B: 1 pt | Late: gr 3 bone fracture, 1 pt | ||||||||||
| Yasuda et al., 2019, NIRS [30] | 57 | 45 Gy/2 fr | 54 (7–103) | Median 3.3 cm | Single: 56 pts | None | A: 51 pts | 1 yr: 98% | NR | 1 yr: 97% | Acute: gr 3 skin toxicity, 2 pts |
| Multiple: 1 pt | B: 6 pts | 3 yr: 91% | 3 yr: 67% | ||||||||
| 5 yr: 91% | 5 yr: 45% | Late: none | |||||||||
| Fujita et al., 2022, NIRS [14] | 69 | 45 Gy/2 fr, 48 Gy/2 fr, 52.8 Gy/4 fr | 51.6 (3.1–130.0) | Median 2.7 cm | Single: 66 pts | NR | A: 68 pts | 2 yr: 92.1% | 2 yr: 77.7% | 2 yr: 83.7% | Acute: none |
| Multiple: 3 pts | B: 1 pt | 5 yr: 89.7% | 5 yr: 50.0% | 5 yr: 55.7% | Late: none | ||||||
| Hiroshima et al., 2023 NIRS [16] | 58 | 45 Gy/2 fr, 48 Gy/2 fr, 52.8 Gy/4 fr, 60 Gy/4 fr | 20.5 (2.7–108) | Median 3.2 cm | Single: 52 tumors | Present in 8 of 69 tumors | B: 58 pts | 1 yr: 96.4% | 1 yr: 38.6% | 1 yr: 80.4% | Acute: gr 3 hepatotoxicity |
| Multiple: 16 tumors | 2 yr: 96.4% | 2 yr: 6.9% | 2 yr: 46.0% | Late: none | |||||||
| Tomizawa et al., 2023, Gunma [29] | 41 | 52.8–60.0 Gy/4–12 fr | 21 (2–88) | Median 3.2 cm | NR | NR | A: 38 pts | 1 yr: 93.4% | 1 yr: 42.1% | 2 yr: 56.0% | Acute or late: gr 3 thrombocytopenia, 1 pt, gr 3 bile duct stenosis 1 pt, gastrointestinal toxicity 2 pts, gr 3 bile duct bleeding, 1 pt |
| B: 3 pts | 2 yr: 83.0% | 2 yr: 16.3% | |||||||||
fr, fraction; gr, grade; HIBMC, Hyogo Ion Beam Medical Center; HIT, Heidelberg Ion Beam Therapy Center; NIRS, National Institute of Radiological Sciences; NR, not reported; Pt, patient; RBE, relative biological effectiveness; SPHIC, Shanghai Proton and Heavy Ion Center; J-CROS, Japan Carbon Ion Radiation Oncology Study Group; yr, year.
REFERENCES
-
METRICS

- ORCID iDs
-
Jinsil Seong

https://orcid.org/0000-0003-1794-5951 - Related articles
-
Recent advances in the management of hepatocellular carcinoma2024 January;30(1)
The prime time for management of hepatocellular carcinoma in Hong Kong2023 April;29(2)
Systemic therapy in advanced hepatocellular carcinoma2023 April;29(2)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



