| Clin Mol Hepatol > Volume 27(1); 2021 > Article |
|
See the commentary-article "Drug-drug interactions with direct-acting antivirals ŌĆö less is more" on page 81.
ABSTRACT
Background/Aims
DirectŌĆÉacting antivirals (DAAs) have been approved for hepatitis C virus (HCV) treatment in patients with end-stage renal disease (ESRD) on hemodialysis. Nevertheless, the complicated comedications and their potential drug-drug interactions (DDIs) with DAAs might limit clinical practice in this special population.
Methods
The number, class, and characteristics of comedications and their potential DDIs with five DAA regimens were analyzed among HCV-viremic patients from 23 hemodialysis centers in Taiwan.
Results
Of 2,015 hemodialysis patients screened in 2019, 169 patients seropositive for HCV RNA were enrolled (mean age, 65.6 years; median duration of hemodialysis, 5.8 years). All patients received at least one comedication (median number, 6; mean class number, 3.4). The most common comedication classes were ESRD-associated medications (94.1%), cardiovascular drugs (69.8%) and antidiabetic drugs (43.2%). ESRD-associated medications were excluded from DDI analysis. Sofosbuvir/velpatasvir/voxilaprevir had the highest frequency of potential contraindicated DDIs (red, 5.6%), followed by glecaprevir/pibrentasvir (4.0%), sofosbuvir/ledipasvir (1.3%), sofosbuvir/velpatasvir (1.3%), and elbasvir/grazoprevir (0.3%). For potentially significant DDIs (orange, requiring close monitoring or dose adjustments), sofosbuvir/velpatasvir/voxilaprevir had the highest frequency (19.9%), followed by sofosbuvir/ledipasvir (18.2%), glecaprevir/pibrentasvir (12.6%), sofosbuvir/velpatasvir (12.6%), and elbasvir/grazoprevir (7.3%). Overall, lipid-lowering agents were the most common comedication class with red-category DDIs to all DAA regimens (n=62), followed by cardiovascular agents (n=15), and central nervous system agents (n=10).
Graphical Abstract
Chronic hepatitis C virus (HCV) infection is one of the leading causes of liver cirrhosis, hepatocellular carcinoma (HCC) and liver-related death. The global prevalence of chronic HCV infections in 2015 was estimated to be 1.0%, corresponding to 71.1 million people [1]. HCV infection is endemic in Taiwan, with estimated prevalence rates of antibodies to HCV (anti-HCV) ranging from 3.3% to 8.6% [2-4], and leads to substantial clinical and economic burden.
Taiwan has the highest prevalence and annual incidence of end-stage renal disease (ESRD) worldwide [5]. Uremic patients on maintenance hemodialysis are at great risk for HCV infection. From 2012 to 2015, the prevalence of HCV infection among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study was nearly 10%, which is much higher than that in the general population [6]. Previous reports indicated that ESRD patients on dialysis with HCV infections have an increased risk of death, hospitalization, anemic complications, and worse quality of life scores than those without HCV infection [7,8]. Given the higher hepatic and extrahepatic adverse outcomes of chronic HCV infection and the benefits associated with HCV viral clearance [9-12], effective treatment and elimination of HCV infection are essential for this specific population.
DirectŌĆÉacting antivirals (DAAs) have become the firstŌĆÉline treatment for HCV infection [13-16]. Compared to interferon-based treatment [17,18], DAA therapy is generally more tolerable, requires a shorter duration, and is more effective. However, the guidelines also highlight the importance of considering and monitoring potential drugŌĆÉdrug interactions (DDIs) between DAAs and comedications [13-16]. To avoid potential DDIs and to optimize patient safety and treatment efficacy, it is important to review all the medications taken by the patient, including over-the-counter preparations and recreational drugs, before and during DAA therapy. Given the large number of potential comedications and limited pharmacokinetic data in ESRD patients [19], DDIs have become a challenge in the era of DAAs in the clinical setting. Several studies have investigated potential DDIs with DAAs among the general population with HCV infection in clinical practice [20-22]. Nevertheless, comorbidities, comedications and potential DDIs in hepatitis C patients with ESRD on hemodialysis remain elusive. Apart from several new DAA regimens, which have been licensed for the treatment of HCV infection, the Food and Drug Administration (FDA) has recently amended the package inserts for sofosbuvir (SOF)-containing regimens to allow use in patients with an estimated glomerular filtration rate (eGFR) Ōēż30 mL/min and those on dialysis, based on validated safety and efficacy [23,24]. Updated information regarding the potential DDIs associated with these regimens is essential. The current study aimed to investigate the frequency of comedications and potential DDIs with DAA regimens in hepatitis C patients on hemodialysis.
All procedures performed in studies involving human participants were in accordance with the ethical standards of Institutional Review Board of Kaohsiung Medical University Chung-Ho Memorial Hospital (IRB No.: KMUHIRB-E(I)-20180325) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
This observational study recruited chronic HCV-infected patients with ESRD on hemodialysis from 23 hemodialysis centers of the Formosan Coalition for the Study of Liver Disease in Chronic Kidney Disease (FORMOSA-LIKE group) in Taiwan between January 2019 and November 2019 [25-27]. The inclusion criteria were as follows: 1) ESRD under maintenance hemodialysis and 2) seropositive for anti-HCV antibodies and HCV RNA. The study was approved by the ethical committee of Kaohsiung Medical University Hospital, and written informed consent was obtained from each participant prior to enrollment. The clinical trial registration number of this study is NCT03803410, and the first posted date is January 14, 2019.
All patients were interviewed, assessed for their anthropomorphic measurements, and had their medical records reviewed at enrollment to capture patient demographics, comorbidities and concurrent medications. HCV RNA and genotypes were measured using a real-time PCR assay (RealTime HCV; Abbott Molecular, Des Plaines, IL, USA) [28]. Concurrent medications were classified into nine major therapeutic classes prespecified by the current study (Table 1). The potential DDIs of each comedication with five interferonŌĆÉfree DAA regimens, including SOF/ledipasvir (LDV), SOF/velpatasvir (VEL), SOF/VEL/voxilaprevir (VOX), elbasvir (EBR)/grazoprevir (GZR), and glecaprevir (GLE)/pibrentasvir (PIB), were analyzed. A vast majority of patients were on ESRD-associated medications, including vitamin supplements, folic acid, calcium carbamide/calcium carbonate, aluminum hydroxide/aluminum acetate, calcitriol/vitamin D, erythropoiesis-stimulating agents, iron supplements, zinc gluconate/zinc oxide, and calcium polystyrene sulfonate. Since the favored ESRD-associated medications varied among hemodialysis centers, all of which did not have clinically significant DDIs with DAA regimens, they were excluded from the DDI evaluation to minimize their interference in the actual frequency of potential DDIs of comedications. Patients who received medications other than ESRD-associated medications were included in the DDI analysis. The DDI analysis was conducted based on known DDIs between DAAs and comedications from the University of Liverpool ŌĆśHEP Drug Interaction CheckerŌĆÖ and ŌĆśLexicomp Drug Interaction CheckerŌĆÖ [29,30]. Medications not included in the HEP Drug Interaction Checker or Lexicomp Drug Interaction Checker were excluded from the DDI analysis due to lack of DDI information. The DDIs were assigned to four risk categories as follows: red, contraindicated (should not be coadministered); orange, potential clinically significant interaction (monitoring and caution required); yellow, potential interaction with weak intensity (monitoring unlikely required); and green, no clinically significant DDI.
Patient demographics and clinical characteristics were summarized using the mean┬▒standard deviation, median (interquartiles), and number (percentage) when appropriate. The comorbidity analysis results were described with numbers and percentages. Similarly, the DDI analysis results were summarized with numbers and percentages. All tests were two-sided. All analyses were performed with the SPSS version 19.0 statistical package (SPSS, Inc., Chicago, IL, USA).
Of 2,015 patients on hemodialysis, 169 patients with HCV viremia were enrolled in the study to analyze comedications and predict their DDIs with DAAs. The clinical characteristics of the patients are listed in Table 2. The mean age was 65.6 years, with 56.2% of patients aged >65 years; 51.5% were male. The median duration of hemodialysis was 5.8 years (interquartile [IQR], 3.0ŌĆō12.6 years), and the most common cause of ESRD was diabetes (54.4%). HCV genotype 2 was the most prevalent genotype (47.9%), followed by HCV genotype 1b (42.0%). Only one patient had prior treatment experience with interferon-based therapies. Baseline laboratory characteristics of the patients are demonstrated in Supplementary Table 1. The median fibrosis-4 (FIB-4) score was 1.81 (IQR, 1.34ŌĆō2.85), and a high proportion of patients had FIB-4 scores lower than 3.25 (n=134, 79.2%). The mean HCV viral load was 5.6┬▒1.2 log IU/mL.
All patients received at least one comedication, with a median comedication number of 6 (IQR, 3ŌĆō9) (Supplementary Table 2). The three most common comedication classes were ESRD-associated medications (94.1%), cardiovascular agents (69.8%), and antidiabetic drugs (43.2%). Among the other agents, liver protectants, including silymarin and ursodeoxycholic acid were most prevalent (n=18, 10.7%). After excluding ESRD-associated medications, 158 patients (93.5%) received at least one comedication, with a median comedication number of 4 (IQR, 2ŌĆō6).
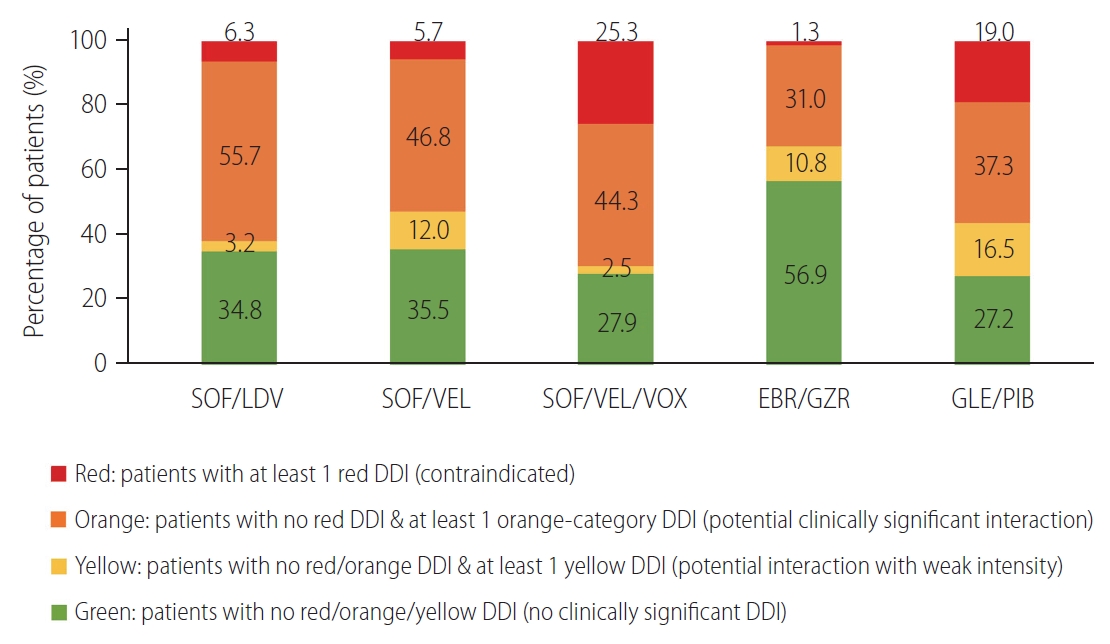
A total of 158 patients who received medications other than ESRD-associated medications were included in the DDI analysis. Figure 1 presents the proportion of patients with the most severe potential DDI category for different DAA regimens. Patients who had at least one comedication with contraindicated (red) DDIs were categorized into the red DDI class. Patients who had no comedication with red-category DDIs but at least one comedication with potential clinically significant (orange) DDIs were classified as the orange DDI class. In the red DDI class, SOF/VEL/VOX was the most prevalent (40 patients, 25.3%), followed by GLE/PIB (30 patients, 19.0%), SOF/LDV (10 patients, 6.3%), SOF/VEL (nine patients, 5.7%) and EBR/GZR (two patients, 1.3%). In addition, the percentage of patients without potential DDIs was higher with EBR/GZR (56.9%) than with the other regimens.
Next, we analyzed the frequency of potential DDIs of each comedication, other than ESRD-associated medications, with each possible DAA regimen (Fig. 2). A total of 755 comedications other than ESRD-associated medications were taken by the 158 patients. The most frequent DDI category was green for each DAA regimen: 77.1% (n=582) for SOF/LDV, 78.9% (n=596) for SOF/VEL, 70.7% (n=534) for SOF/VEL/VOX, 88.5% (n=668) for EBR/GZR, and 71.6% (n=541) for GLE/PIB. SOF/VEL/VOX had the highest frequency of red-category DDIs (5.6%, n=42), followed by GLE/PIB at 4.0%, SOF/LDV and SOF/VEL at 1.3%, and EBR/GZR at 0.3%. The highest frequency of orange-category DDIs was 19.9% with SOF/VEL/VOX, followed by 18.2% with SOF/LDV, 12.6% with GLE/PIB and SOF/VEL, and 7.3% with EBR/GZR.
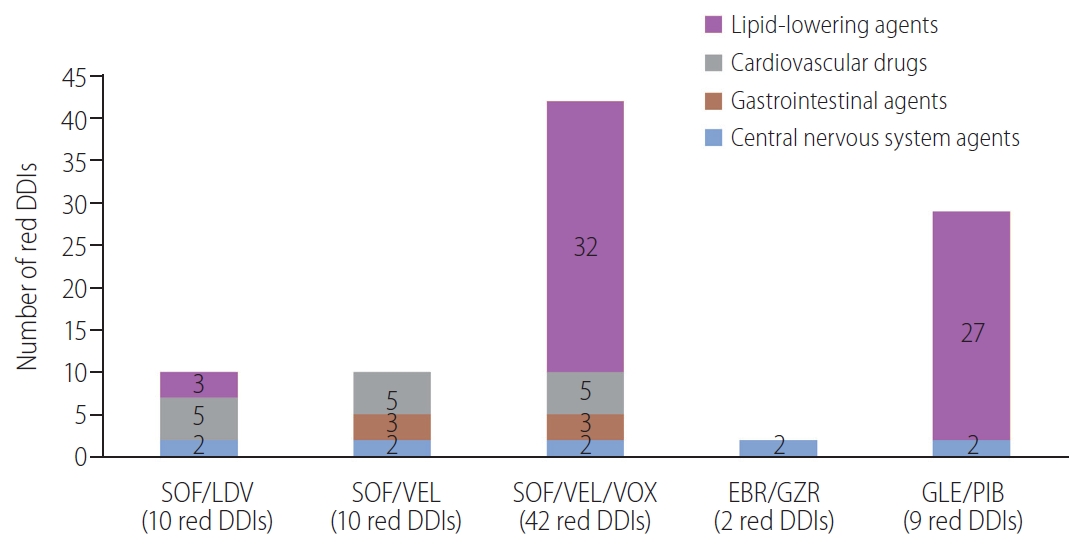
Then, we evaluated the number of comedications with red-category DDIs in each class (Fig. 3). SOF/VEL/VOX and GLE/PIB had a much higher number of red-category DDIs (42 and 29, respectively), which was contraindicated mainly with lipidŌĆÉlowering agents (32 and 27, respectively). EBR/GZR had the fewest potential red-category DDIs, which were with central nervous system (CNS) agents. Overall, lipid-lowering agents were the most common comedication class with red-category DDIs to all DAA regimens (n=62), followed by cardiovascular agents (n=15), CNS agents (n=10), and gastrointestinal agents (n=6). Amiodarone was the only cardiovascular drug with contraindicated DDIs for SOF/LDV, SOF/VEL, and SOF/VEL/VOX in the current study, given the risk of symptomatic bradycardia (Supplementary Table 3).
Supplementary Figure 1 shows the number of orange-category DDIs. SOF/VEL/VOX had the highest number of potential orange-category DDIs (n=150), which were predominantly associated with cardiovascular drugs and antidiabetic drugs. In contrast, EBR/GZR had the fewest number of orange-category DDIs (n=55), and these DDIs were mainly caused by lipidŌĆÉlowering agents. Overall, cardiovascular agents were the most common comedication class with orange-category DDIs to all DAA regimens (n=210), followed by gastrointestinal agents (n=115), lipid-lowering agents (n=104), and antidiabetic drugs (n=80).
To the best of our knowledge, this is the first realŌĆÉworld study to investigate comedications and potential DDIs with DAAs in hepatitis C patients with ESRD on hemodialysis. In the current study, we demonstrated that 93.5% of hepatitis C patients on hemodialysis had at least one comedication other than ESRD-associated medications. The chance of having a contraindicated DDI was much higher, at 19ŌĆō25.3%, if the patients were commencing SOF/VEL/VOX or GLE/PIB than if the patients were taking EBR/GZR, SOF/LDV, or SOF/VEL, at only 1.3ŌĆō6.3%. Of the 755 comedications other than ESRD-associated medications in 158 patients, lipid-lowering agents (n=62) were the most common comedication class with contraindicated DDIs to all DAA regimens, and cardiovascular agents (n=210) were the most common comedication class with potential clinically significant DDIs to all DAA regimens.
The present study included an elderly population with a mean age of 65.6 years, which is similar to the general population of HCV patients in Taiwan and Japan [21,31]. Nevertheless, the proportion of patients taking at least one comedication other than dialysis-associated drugs (93.5%) among our hemodialysis patients was higher than that in two studies of the general population (75.7% and 41.9%) [21,31]. This difference is due to multiple comorbidities in hemodialysis patients. Concurrent medications were widely used by hemodialysis patients in the current study, with a median number of comedications of 6 and a mean number of comedication classes of 3.4 per person. After excluding ESRD-associated medications, the median number of comedications was 4, and the mean number of comedication classes was 2.5 per patient. Cardiovascular agents, antidiabetic drugs, and gastrointestinal agents were the most common comedications taken by patients in the current study. This result may be attributed to the high prevalence of comorbidities, including hypertension, diabetes, ischemic heart disease and hyperlipidemia in elderly patients and ESRD patients [32]. Increased incidences of digestive diseases, hypertension and diabetes have also been reported in hepatitis C patients [21,31,33].
In the current study, we assessed the potential DDIs of five widely used DAA regimens, including sofosbuvir-based regimens. Sofosbuvir-based regimens were not recommended for patients with an eGFR <30 mL/min or those on dialysis because of concerns of increased plasma concentrations of the primary sofosbuvir metabolite GS-331007 and unvalidated drug safety and efficacy. Recently, several studies have demonstrated the safety and efficacy of these drugs for this special population [23,24]. Thus, the FDA approved sofosbuvir-containing regimens for HCV patients with severe renal impairment and ESRD in November 2019. Understanding the potential DDIs of sofosbuvir-based therapy for ESRD patients is therefore clinically important, especially for the red (contraindicated, should not be coadministered) and orange (potential clinically significant interaction, monitoring and caution required) DDI categories. In addition to renal function, a patientŌĆÖs liver function is another concern relating to therapeutic regimen selection. DAAs containing nonstructural 3/4A protease inhibitors are contraindicated for patients with decompensated liver cirrhosis. In the present study, most patients did not have advanced liver fibrosis, which was defined as FIB-4 score Ōēź3.25 [34]. Only six patients (3.6%) had liver cirrhosis, which was defined as FIB-4 score Ōēź6.5. None of them had liver decompensation.
The proportion of patients with at least one potential red-category DDI was higher among those taking SOF/VEL/VOX (25.3%) or GLE/PIB (19.0%) but was much lower among those taking EBR/GZR (1.3%), SOF/LDV (6.3%) or SOF/VEL (5.7%). We also analyzed the prevalence of potential DDIs for each possible DAA regimen. SOF/VEL/VOX had the highest proportion of red-category DDIs and orange-category DDIs, followed by GLE/PIB, while EBR/GZR had the lowest proportion of red-category DDIs and orange-category DDIs among the five DAA regimens. The high prevalence of significant DDIs with SOF/VEL/VOX may be ascribed to the more components of the HCV nonstructural protein 3/4A (NS3/4A) inhibitor (voxilaprevir), NS5B inhibitor (sofosbuvir) and NS5A inhibitor (velpatasvir). However, SOF/VEL/VOX is mainly recommended for patients who fail prior DAA therapy [16]. Given that current DAA therapies have high sustained virologic response rates (95ŌĆō99%) for DAA-naive HCV patients, SOF/VEL/VOX is rarely used in clinical practice. Among the pangenotypic regimens currently recommended for DAA-naive patients, SOF/VEL had the lowest rate of DDIs.
The distribution of each drug class with potential contraindicated DDIs was also assessed. The potential red-category DDIs were mainly associated between cardiovascular drugs and SOF/LDV and SOF/VEL, lipidŌĆÉlowering agents and SOF/VEL/VOX and GLE/PIB, and CNS agents and EBR/GZR. Amiodarone was the only cardiovascular drug with contraindicated DDIs for sofosbuvir-based DAAs, given the risk of symptomatic bradycardia [35-38]. Although the mechanism of this effect remains unknown, we should avoid the coadministration of amiodarone and sofosbuvir-based DAAs, especially in subjects taking beta blockers or those with underlying cardiac comorbidities. Another class of comedications with the most contraindicated DDIs is lipid-lowering agents. Increased serum concentrations of lipid-lowering agents and statin-related myopathy may be induced by the inhibition of organic anion transporting polypeptide (OATP)1B1 and OATP1B3, breast cancer resistance protein, or cytochrome P450 (CYP) 3A4 [36,38,39]. The CNS agents with contraindicated DDIs in this study were phenytoin and oxcarbazepine. The induction of CYP3A4 and P-glycoprotein by these agents may significantly decrease plasma concentrations of DAAs and result in a loss of efficacy and potential therapeutic failure. Given the high prevalence of comedications in hemodialysis patients, our study provides physicians and pharmacists with useful information to select appropriate DAA regimens with fewer potential DDIs and reduce the time required to review comedications and DDIs.
For certain comedications with red/orange-category DDIs, such as lipid-lowering agents, antihypertensive medications, anti-diabetic drugs, and acid-reducing agents (e.g., proton pump inhibitors [PPIs], H2 receptor antagonists, and antacids), it can be relatively straightforward to switch to appropriate alternative regimens or discontinue the treatment given the short duration of DAA therapies [40]. Other strategies for the management of DDIs include increasing monitoring, decreasing dose, altering administration time, and separating medication or administration. Lipid-lowering agents are frequent comedications with potential DDIs to DAAs in uremic HCV patients. For HCV genotype 1 or 4 infected patients on lipid-lowering agents, EBR/GZR with lipid-lowering agents, such as ezetimibe, pitavastatin or pravastatin may be recommended to avoid red-category DDIs and to maintain serum lipid levels. Alternatively, SOF/VEL with lipid-lowering agents, such as ezetimibe and pravastatin, might be another optimal regimen for all HCV genotypes. Nevertheless, careful monitoring for adverse events, such as myopathy and rhabdomyolysis, and dose reduction of statins, if needed, are mandatory in clinical practice. For patients without a history or risk of cardiovascular complications, lipid-lowering agents might be held for 8ŌĆō12 weeks with close monitoring of serum lipid profiles for potential lipid rebound and risk of significant cardiovascular events [41]. Several PPIs are categorized as having orange-category DDIs to SOF/LDV, SOF/VEL, and SOF/VEL/VOX due to pH-dependent solubility issues. In accordance with the prescribing information, SOF/LDV and SOF/VEL/VOX can be administered simultaneously with a low-dose PPI (defined as daily dose comparable to 20 mg of omeprazole or lower). SOF/VEL can be coadministered with a low-dose PPI when given 4 hours before the PPI [36-38]. However, this kind of adjustment may not be suitable for all comedications. For example, switching an anticonvulsant to an appropriate alternative may be complex. Apart from the disease state being treated and specific patient factors, the switch can involve specific titration schedules and overlap, requiring specific monitoring and management [40]. In this situation, it may be more suitable to choose a different DAA regimen with fewer interactions instead of switching the comedications.
There are several limitations to our study. First, our study investigated potential DDIs between DAAs and comedications, rather than actual DDIs in clinical practice. This methodology may limit the analyses of the efficacy of DAA treatments in this special population. However, previous reports have demonstrated that DAAs are effective for HCV patients with ESRD [23,24,42]. Second, herbal medicines and supplements taken by our patients were not recorded or analyzed. Given the high prevalence of herbal medicine and supplement use in the hemodialysis population [43], the potential DDIs with DAA regimens deserve more attention. Since the University of Liverpool HEP Drug Interaction Checker and Lexicomp Drug Interaction Checker do not include herbal medicines and supplements, there is currently no available tool to guide physicians in assessing the potential DDIs between herbal medicines and DAA regimens; thus, physicians should recommend that their patients stop using these products during DAA therapy. Third, comedications varied among study sites. However, under the authority of the National Health Insurance of Taiwan, each hemodialysis center could access all the prescribed medications reimbursed by the National Health Insurance with a nation-based cloud system, which could provide almost complete information on the comedications. Finally, some common ESRD-associated medications were not used in the study cohort. These medications included cinacalcet (for hyperparathyroidism), sevelamer, lanthanum carbonate, and ferric citrate (phosphate binders). Similar to the ESRD-associated medications taken by our patients, almost all of them have no clinically significant DDIs with the five DAA regimens, except for sevelamer, which has potential weak interactions with SOF/LDV, SOF/VEL, SOF/VEL/VOX, and GLE/PIB.
In conclusion, hepatitis C patients with ESRD on hemodialysis had a high prevalence of comedication use. The potential DDIs between these comedications and DAA regimens differed, with the most potential DDIs occurring with SOF/VEL/VOX and the fewest potential DDIs occurring with EBR/GZR. A careful assessment of the patientŌĆÖs concurrent medications and a comprehensive evaluation of their potential DDIs with each DAA regimen are essential to selecting the appropriate DAA regimen and optimizing safety and efficacy.
ACKNOWLEDGMENTS
We thank the participation of the members of the Formosan Coalition for the study of Liver Disease in Chronic Kidney Disease (FORMOSA-LIKE group): Dr. Lii-Jia Yang, Kaohsiung Municipal Cijin Hospital; Dr. Ming-Hsing Sung, Hsing-Yi Clinic; Dr. Shih-Pi Lin, Lenity Clinic; Dr. Fei-Ching Li, Yu-Sheng Clinic; Dr. Jheng-Tai Shien, Chung-Ching Clinic; Dr. Chen-Hung Shih, Wu-Fu Clinic; Dr. June-Ming Yang, June-Ming Clinic; Dr. Cheng-Hsueh Lee, Ming-Gang Clinic; Dr. Yi-Cheng Chen, Shih-Chuan Clinic; Dr. Yen-chao Wang, Gang-Shan Clinic; Dr. Meng-Chang Yang, Park Medicines Clinic; Dr. June-Tse Yang, Yung Ho General Hospital; Dr. Pei-Ing Kong, Yong Ding Clinic; Dr. Hsuan-Sheng Chuang, Shun Tai Clinic; Dr. Chi-Chin Wu, Shinkao Hospital; Dr. Shih-Meng Yeh, You Zhen Clinic; Dr. Tung-Chang Chuang, People Clinic; Dr. Ying-Chih Lin, PingTung Hospital and Dr. Jer-Ming Chang, Wen-Hsiung Hospital. The authors thank secretary help from Taiwan Liver Research Foundation (TLRF).
FOOTNOTES
AuthorsŌĆÖ contribution
Conception and design: Yi-Wen Chiu, Ming-Lung Yu
Data collection: all authors
Data analysis and interpretation: Po-Yao Hsu, Yi-Wen Chiu, Ming-Lung Yu
Manuscript drafting or critical revising: all authors
All named authors have read and approved the final version of the manuscript
Conflicts of Interest: Ming-Lung Yu has served as a speaker for AbbVie, Abbott, Ascletis, BristolŌĆÉMyers Squibb, Gilead, Merck, a consultant for AbbVie, Abbott, Ascletis, BristolŌĆÉMyers Squibb, Gilead, Merck and PharmaEssentia, and has received research funding from AbbVie, Abbott, BristolŌĆÉMyers Squibb, Gilead, and Merck. Chung-Feng Huang has served as a speaker for AbbVie, Abbott, BristolŌĆÉMyers Squibb, Gilead, and Merck.
SUPPLEMENTAL MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
Supplementary┬ĀTable┬Ā3.
Red-category drug-drug interactions between HCV DAAs and comedications
Supplementary┬ĀFigure┬Ā1.
Number of potential orange-category drug-drug interactions (DDIs) in each drug class for each possible direct-acting antiviral (DAA) regimen. SOF, sofosbuvir; LDV, ledipasvir; VEL, velpatasvir; VOX, voxilaprevir; EBR, elbasvir; GZR, grazoprevir; GLE, glecaprevir; PIB, pibrentasvir.
Figure┬Ā1.
Proportion of patients with the most severe potential drug-drug interactions (DDIs) for each possible direct-acting antiviral (DAA) regimen (n=158). SOF, sofosbuvir; LDV, ledipasvir; VEL, velpatasvir; VOX, voxilaprevir; EBR, elbasvir; GZR, grazoprevir; GLE, glecaprevir; PIB, pibrentasvir.
Figure┬Ā2.
Frequency of potential drug-drug interactions (DDIs) of each comedication, other than end-stage renal disease-associated medications, with each possible direct-acting antiviral (DAA) regimen (number of interactions, 755). SOF, sofosbuvir; LDV, ledipasvir; VEL, velpatasvir; VOX, voxilaprevir; EBR, elbasvir; GZR, grazoprevir; GLE, glecaprevir; PIB, pibrentasvir.

Figure┬Ā3.
Number of potential red-category drug-drug interactions (DDIs) in each drug class for each possible direct-acting antiviral (DAA) regimen. SOF, sofosbuvir; LDV, ledipasvir; VEL, velpatasvir; VOX, voxilaprevir; EBR, elbasvir; GZR, grazoprevir; GLE, glecaprevir; PIB, pibrentasvir.
Table┬Ā1.
Therapeutic drug classes of comedication in HCV-viremic patients with ESRD under hemodialysis
Table┬Ā2.
Baseline patient demographic characteristics and clinical features
Abbreviations
anti-HCV
antibodies to hepatitis C virus
CNS
central nervous system
CYP
cytochrome P450
DAAs
direct-acting antivirals
DDIs
drug-drug interactions
EBR
elbasvir
eGFR
estimated glomerular filtration rate
ESRD
end-stage renal disease
FDA
Food and Drug Administration
FIB-4
fibrosis-4
FORMOSA-LIKE group
Formosan Coalition for the Study of Liver Disease in Chronic Kidney Disease
GLE
glecaprevir
GZR
grazoprevir
HCC
hepatocellular carcinoma
HCV
hepatitis C virus
IQR
interquartile
LDV
ledipasvir
OATP
organic anion transporting polypeptide
PIB
pibrentasvir
PPIs
proton pump inhibitors
SOF
sofosbuvir
VEL
velpatasvir
VOX
voxilaprevir
REFERENCES
1. Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2017;2:161-176.

2. Yu ML, Yeh ML, Tsai PC, Huang CI, Huang JF, Huang CF, et al. Huge gap between clinical efficacy and community effectiveness in the treatment of chronic hepatitis C: a nationwide survey in Taiwan. Medicine (Baltimore) 2015;94:e690.


3. Yang JF, Lin CI, Huang JF, Dai CY, Lin WY, Ho CK, et al. Viral hepatitis infections in southern Taiwan: a multicenter community-based study. Kaohsiung J Med Sci 2010;26:461-469.


4. Chen CH, Yang PM, Huang GT, Lee HS, Sung JL, Sheu JC. Estimation of seroprevalence of hepatitis B virus and hepatitis C virus in Taiwan from a large-scale survey of free hepatitis screening participants. J Formos Med Assoc 2007;106:148-155.


5. Saran R, Robinson B, Abbott KC, Agodoa LYC, Bragg-Gresham J, Balkrishnan R, et al. US renal data system 2018 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2019;73(3 Suppl 1):A7-A8.


6. Jadoul M, Bieber BA, Martin P, Akiba T, Nwankwo C, Arduino JM, et al. Prevalence, incidence, and risk factors for hepatitis C virus infection in hemodialysis patients. Kidney Int 2019;95:939-947.


7. Fabrizi F, Dixit V, Messa P. Impact of hepatitis C on survival in dialysis patients: a link with cardiovascular mortality? J Viral Hepat 2012;19:601-607.


8. Goodkin DA, Bieber B, Jadoul M, Martin P, Kanda E, Pisoni RL. Mortality, hospitalization, and quality of life among patients with hepatitis C infection on hemodialysis. Clin J Am Soc Nephrol 2017;12:287-297.



9. S├Čderholm J, Millbourn C, B├╝sch K, K├Čvamees J, Schvarcz R, Lindahl K, et al. Higher risk of renal disease in chronic hepatitis C patients: antiviral therapy survival benefit in patients on hemodialysis. J Hepatol 2018;68:904-911.


10. Yu ML, Huang CF, Yeh ML, Tsai PC, Huang CI, Hsieh MH, et al. Time-degenerative factors and the risk of hepatocellular carcinoma after antiviral therapy among hepatitis C virus patients: a model for prioritization of treatment. Clin Cancer Res 2017;23:1690-1697.


11. Huang JF, Yu ML, Lee CM, Dai CY, Hou NJ, Hsieh MY, et al. Sustained virological response to interferon reduces cirrhosis in chronic hepatitis C: a 1,386-patient study from Taiwan. Aliment Pharmacol Ther 2007;25:1029-1037.


12. Huang CF, Lai HC, Chen CY, Tseng KC, Kuo HT, Hung CH, et al. Extrahepatic malignancy among patients with chronic hepatitis C after antiviral therapy: a real-world nationwide study on Taiwanese chronic hepatitis C cohort (T-COACH). Am J Gastroenterol 2020;115:1226-1235.


13. Omata M, Kanda T, Wei L, Yu ML, Chuang WL, Ibrahim A, et al. APASL consensus statements and recommendation on treatment of hepatitis C. Hepatol Int 2016;10:702-726.



14. European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol 2018;69:461-511.


15. Yu ML, Chen PJ, Dai CY, Hu TH, Huang CF, Huang YH, et al. 2020 Taiwan consensus statement on the management of hepatitis C: part (I) general population. J Formos Med Assoc 2020;119:1019-1040.


16. Yu ML, Chen PJ, Dai CY, Hu TH, Huang CF, Huang YH, et al. 2020 Taiwan consensus statement on the management of hepatitis C: part (II) special populations. J Formos Med Assoc 2020;119:1135-1157.


17. Liu CH, Huang CF, Liu CJ, Dai CY, Liang CC, Huang JF, et al. Pegylated interferon-╬▒2a with or without low-dose ribavirin for treatment-naive patients with hepatitis C virus genotype 1 receiving hemodialysis: a randomized trial. Ann Intern Med 2013;159:729-738.


18. Liu CH, Liu CJ, Huang CF, Lin JW, Dai CY, Liang CC, et al. Peginterferon alfa-2a with or without low-dose ribavirin for treatment-naive patients with hepatitis C virus genotype 2 receiving haemodialysis: a randomised trial. Gut 2015;64:303-311.


19. Chiu YW, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol 2009;4:1089-1096.



20. H├Čner Zu Siederdissen C, Maasoumy B, Marra F, Deterding K, Port K, Manns MP, et al. Drug-drug interactions with novel all oral interferon-free antiviral agents in a large real-world cohort. Clin Infect Dis 2016;62:561-567.


21. Liu CH, Yu ML, Peng CY, Hsieh TY, Huang YH, Su WW, et al. Comorbidities, concomitant medications and potential drug-drug interactions with interferon-free direct-acting antiviral agents in hepatitis C patients in Taiwan. Aliment Pharmacol Ther 2018;48:1290-1300.


22. Sicras Mainar A, Navarro Artieda R, Hern├Īndez I, Morillo R. Prevalence of the potential drug-drug interactions between pangenotypic direct-acting antivirals and the concomitant medications associated with patients with chronic hepatitis C virus infection in Spain. Gastroenterol Hepatol 2019;42:465-475.


23. Desnoyer A, Pospai D, L├¬ MP, Gervais A, Heurgu├®-Berlot A, Laradi A, et al. Pharmacokinetics, safety and efficacy of a full dose sofosbuvir-based regimen given daily in hemodialysis patients with chronic hepatitis C. J Hepatol 2016;65:40-47.


24. Borgia SM, Dearden J, Yoshida EM, Shafran SD, Brown A, Ben-Ari Z, et al. Sofosbuvir/velpatasvir for 12 weeks in hepatitis C virus-infected patients with end-stage renal disease undergoing dialysis. J Hepatol 2019;71:660-665.


25. Yu ML, Dai CY, Huang CF, Lee JJ, Yeh ML, Yeh SM, et al. High hepatitis B virus surface antigen levels and favorable interleukin 28B genotype predict spontaneous hepatitis C virus clearance in uremic patients. J Hepatol 2014;60:253-259.


26. Huang CF, Yeh ML, Lee JJ, Chen MC, Dai CY, Huang JF, et al. Hepatitis C viremia interferes with serum hepatitis B virus surface antigen and DNA levels in hepatitis B uremics. Hepatol Int 2014;8:224-232.


27. Chang JM, Huang CF, Chen SC, Dai CY, Yeh ML, Huang JF, et al. Discrepancy between serological and virological analysis of viral hepatitis in hemodialysis patients. Int J Med Sci 2014;11:436-441.



28. Vermehren J, Yu ML, Monto A, Yao JD, Anderson C, Bertuzis R, et al. Multi-center evaluation of the Abbott RealTime HCV assay for monitoring patients undergoing antiviral therapy for chronic hepatitis C. J Clin Virol 2011;52:133-137.


29. University of Liverpool. Interaction charts for directly acting antivirals & ribavirin. University of Liverpool web site, <https://www.hepdruginteractions.org/checker>. Accessed 22 Dec 2019.
30. A Drug-Drug, Drug-Herb, and Herb-Herb analysis tool, provided by Wolters Kluwer Clinical Drug Information utilizing Lexicomp clinical content. UpToDate web site, <https://www.uptodate.com/drug-interactions/#di-druglist>. Accessed 22 Dec 2019.
31. Ruzicka DJ, Tetsuka J, Fujimoto G, Kanto T. Comorbidities and comedications in populations with and without chronic hepatitis C virus infection in Japan between 2015 and 2016. BMC Infect Dis 2018;18:237.




32. Wu MY, Wu MS. Taiwan renal care system: a learning health-care system. Nephrology (Carlton) 2018;23 Suppl 4:112-115.



33. Louie KS, St Laurent S, Forssen UM, Mundy LM, Pimenta JM. The high comorbidity burden of the hepatitis C virus infected population in the United States. BMC Infect Dis 2012;12:86.




34. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317-1325.


35. Food and Drug Administration (FDA). Sovaldi: prescribing information. FDA web site, <https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/204671s002lbl.pdf>. Accessed 22 Dec 2019.
36. Food and Drug Administration (FDA). Harvoni: prescribing information. FDA web site, <https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205834s000lbl.pdf>. Accessed 22 Dec 2019.
37. Food and Drug Administration (FDA). Epclusa: prescribing information. FDA web site, <https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208341s000lbl.pdf>. Accessed 22 Dec 2019.
38. Food and Drug Administration (FDA). Vosevi: prescribing information. FDA web site, <https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209195s000lbl.pdf>. Accessed 22 Dec 2019.
39. Food and Drug Administration (FDA). Maviret: prescribing information. FDA web site, <https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209394s000lbl.pdf>. Accessed 22 Dec 2019.
40. Langness JA, Nguyen M, Wieland A, Everson GT, Kiser JJ. Optimizing hepatitis C virus treatment through pharmacist interventions: identification and management of drug-drug interactions. World J Gastroenterol 2017;23:1618-1626.



41. Huang CF, Dai CY, Yeh ML, Huang CI, Lee HC, Lai WT, et al. Cure or curd: modification of lipid profiles and cardio-cerebrovascular events after hepatitis C virus eradication. Kaohsiung J Med Sci 2020;36:920-928.



- TOOLS
-
METRICS

- ORCID iDs
-
Yi-Wen Chiu

https://orcid.org/0000-0001-6840-5440Ming-Lung Yu

https://orcid.org/0000-0001-8145-1900 - Related articles
-
Drug-drug interactions with direct-acting antivirals ŌĆö less is more2021 January;27(1)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement1
Supplement1 Print
Print



