Impact of everolimus on survival after liver transplantation for hepatocellular carcinoma
Article information
Abstract
Background/Aims
This study aimed to investigate whether everolimus (EVR) affects long-term survival after liver transplantation (LT) in patients with hepatocellular carcinoma (HCC).
Methods
The data from 303 consecutive patients with HCC who had undergone LT from January 2012 to July 2018 were retrospectively reviewed. The patients were divided into two groups: 1) patients treated with EVR in combination with calcineurin inhibitors (CNIs) (EVR group; n=114) and 2) patients treated with CNI-based therapy without EVR (non-EVR group; n=189). Time to recurrence (TTR) and overall survival (OS) after propensity score (PS) matching were compared between the groups, and prognostic factors for TTR and OS were evaluated.
Results
The EVR group exhibited more aggressive tumor biology than the non-EVR group, such as a higher number of tumors (P=0.003), a higher prevalence of microscopic vascular invasion (P=0.017) and exceeding Milan criteria (P=0.029). Compared with the PS-matched non-EVR group, the PS-matched EVR group had significantly better TTR (P<0.001) and OS (P<0.001). In multivariable analysis, EVR was identified as an independent prognostic factor for TTR (hazard ratio [HR], 0.248; P=0.001) and OS (HR, 0.145; P<0.001).
Conclusions
Combined with CNIs, EVR has the potential to prolong long-term survival in patients undergoing LT for HCC. These findings warrant further investigation in a well-designed prospective study.
Graphical Abstract
INTRODUCTION
Concurrent with the major evolution of surgical techniques and immunosuppressant regimens, liver transplantation (LT) criteria for patients with hepatocellular carcinoma (HCC) have rapidly expanded in the past few decades [1-3]. Despite the scarcity of donor organs, LT is theoretically the best treatment option for patients with early stage HCC [4]. However, tumor recurrence remains a major cause of post-transplant death [5]. Unfortunately, no adjuvant therapy has been shown to improve survival after LT for HCC.
Several tumor-related risk factors for HCC recurrence, such as high alpha-fetoprotein (AFP) levels [6,7], poor tumor differentiation [8,9], and the presence of microscopic vascular invasion (MiVI) [10,11], have been extensively demonstrated to be strong risk factors for poor outcomes after LT. Immunosuppression-related risk factors for tumor recurrence have also been reported. Vivarelli et al. [12,13] noted that overexposure to calcineurin inhibitors (CNIs; tacrolimus and cyclosporin) increased the risk of HCC recurrence after LT. Conversely, in a recent systematic review, Grigg et al. [14] reported that mammalian target of rapamycin inhibitors (mTORi)-based immunosuppression might reduce recurrence rates compared with standard CNI-based therapy. Accordingly, mTORi have been considered an alternative immunosuppressive agent to CNIs.
However, despite the expanding use of mTORi-based therapy, it remains unclear whether mTORi have a direct oncologic advantage or whether their benefits simply reflect the indirect effects of reducing CNI levels. Furthermore, no universally accepted guidelines exist for mTORi usage after LT for HCC. Immunosuppression schedules that vary considerably between centers and the wide range of target trough blood levels make it difficult to verify the long-term oncologic effects of mTORi.
At our institution, we have been following a unified immunosuppressive protocol for mTORi usage for several years. In the present study, we reviewed our experience with mTORi (particularly everolimus [EVR]) after LT in patients with HCC to evaluate the impact of EVR on long-term outcomes, specifically time to recurrence (TTR) and overall survival (OS). We also investigated general prognostic factors for TTR and OS after LT for HCC.
MATERIALS AND METHODS
Patient and data collection
This study was approved by the institutional review board of Severance Hospital (reference number, 4-2020-0808). The data were prospectively recorded in our institution’s clinical database. The data from consecutive patients who had undergone LT for HCC from January 2012 to July 2018 at Severance Hospital, Seoul, Korea, were retrospectively reviewed. All the patients had histologically confirmed pure HCC; we excluded patients with mixed HCC-cholangiocarcinoma tumors. Patients who had died within 1 month after LT and patients who had withdrawn from the protocol during treatment because of intolerable side effects from the drugs (CNI or EVR) were excluded. Information regarding patient demographics (recipient age and sex) and other clinicopathologic features (underlying liver disease, preoperative blood AFP level, model for end-stage liver disease [MELD] score, graft type, fulfillment of Milan criteria, tumor size and number, and MiVI) were extracted from the database. All the resected specimens were subjected to histologic analysis.
Changes in the immunosuppression protocols over time
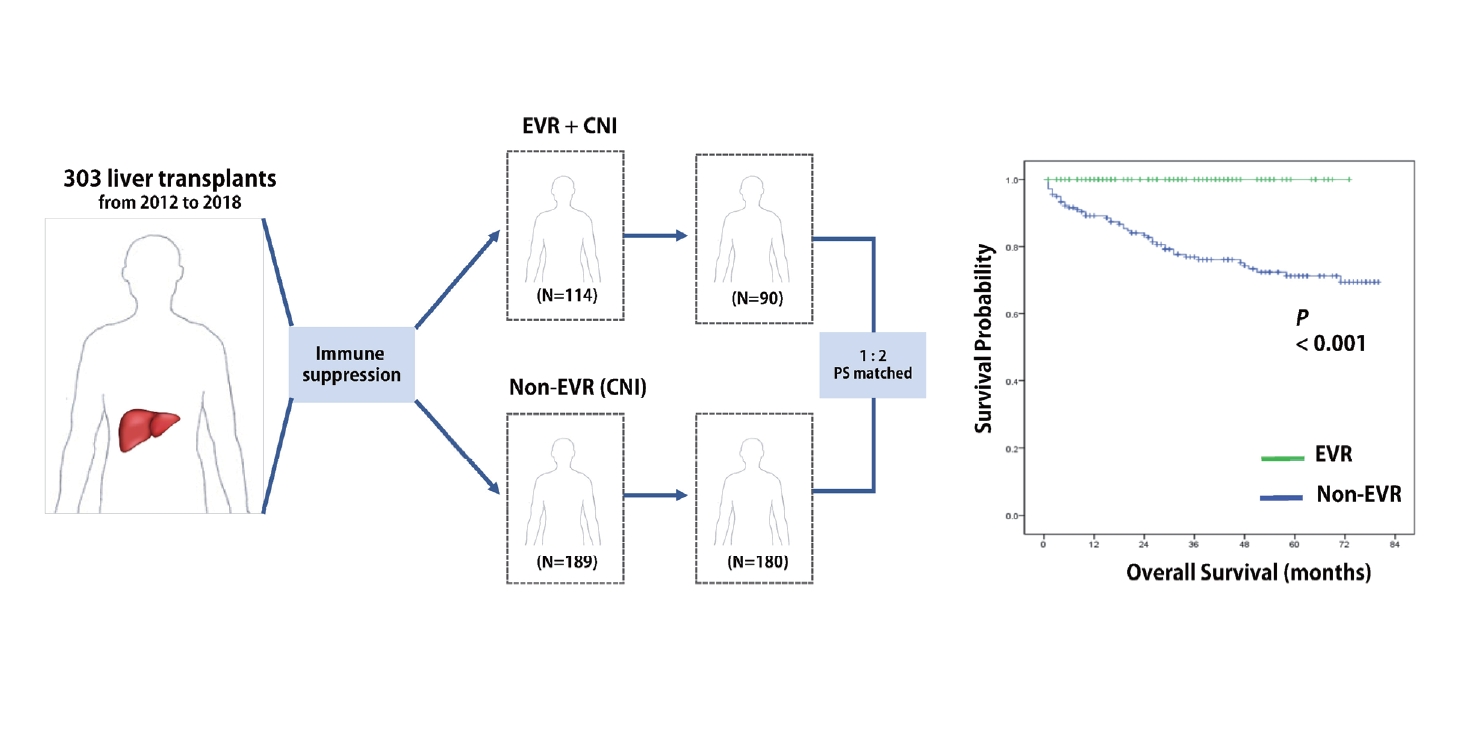
At our institution, all the patients who had undergone LT before 2012 were treated with CNIs as their primary immunosuppressive regimen. However, with increasing recognition of mTORi and their prognostic relevance for survival, we began to use EVR in combination with CNIs in January 2012. During the initial combination period (January 2012 to May 2015), because of our limited experience with EVR, we used EVR only in high-risk patients with unfavorable prognostic factors for tumor recurrence, such as patients exceeding the Milan criteria or those with pathologically confirmed MiVI. Since June 2015, EVR has been covered by Korean National Insurance for combination treatment with tacrolimus after LT, allowing us to gradually expand the use of EVR to low-risk patients. For this study, we divided patients into two groups according to EVR usage: non-EVR group (CNI alone or CNI plus mycophenolate mofetil) and EVR group (CNI plus EVR) (Fig. 1).
Differences in target trough blood levels between the non-EVR and EVR groups
Our immunosuppressive protocol for patients with HCC undergoing LT is shown in Figure 2. Induction immunosuppression therapy was performed with basiliximab injected on the day of LT and postoperative day 4 in all recipients. Two high-dose boluses of methylprednisolone (500 mg) were infused intravenously immediately following portal vein and hepatic artery reperfusion during LT. After LT, intravenous methylprednisolone was tapered to 60 mg/day over 4 days and then switched to oral prednisolone (30 mg/day) on day 5. Oral prednisolone was gradually tapered, reaching 10 mg/day at 4 weeks post-LT. Maintenance immunosuppression comprised tacrolimus-based immunosuppression with or without EVR. EVR was usually initiated at 1 mg twice per day starting at 4 weeks after LT. The target trough blood level of EVR was 3–5 ng/mL. The target trough level of tacrolimus without EVR (non-EVR group) was the conventional range of 5–8 ng/mL throughout the study, while the target trough level for the reduced tacrolimus dose (EVR group) was 3–5 ng/mL after initiating EVR.
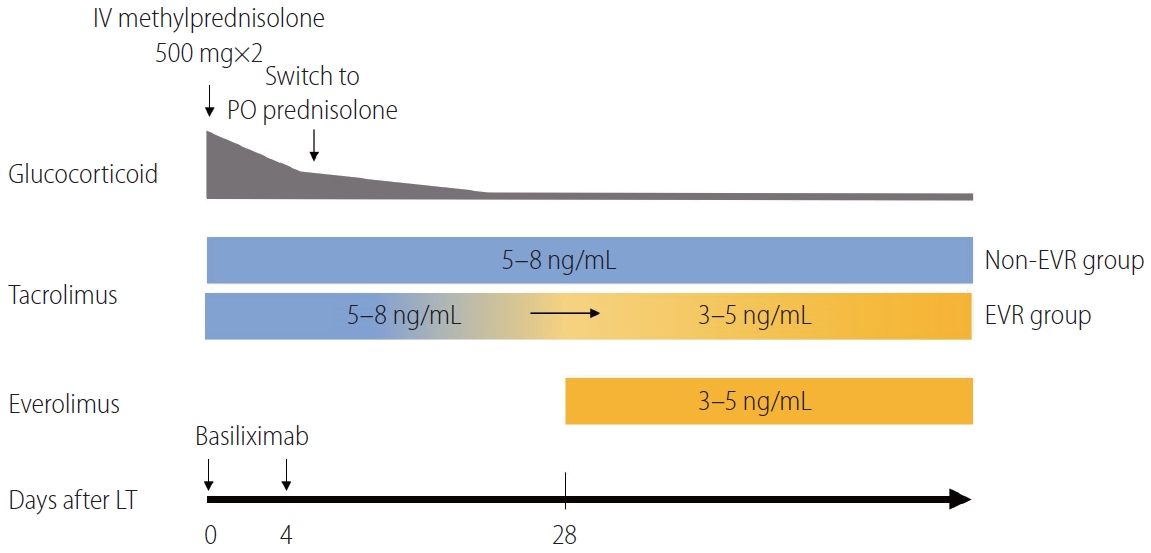
Immunosuppression protocol after liver transplantation in patients with hepatocellular carcinoma. The target blood trough level of tacrolimus for the non-EVR group was maintained at 5–8 ng/mL (blue bar). The target blood trough level of tacrolimus for the EVR group was changed from 5–8 ng/mL to 3–5 ng/mL after EVR was initiated (orange bar). IV, intravenous; PO, oral; EVR, everolimus; LT, liver transplantation.
Post LT follow-up
All the patients were followed up according to our institutional post-LT protocol. All the patients were followed monthly to assess their immunosuppressive drug trough levels until 12 months after discharge. According to those results, the dosage of the CNI or EVR was adjusted to the target trough level. Imaging studies, including dynamic computed tomography and/or ultrasound, were performed every 3 to 6 months after LT. Measurement of the serum AFP levels was also followed by imaging studies. Tumor recurrence was defined as suspicious imaging findings or a pathologically confirmed tumor from biopsy or surgery. The primary endpoint of this study was TTR, while the secondary endpoint was OS. TTR was defined as the time from the date of LT to the time of recurrence. Patients with no recurrent disease were censored at the last time at which they were known to be recurrence-free. OS was defined as the duration from the date of LT to patient death or the study end date.
Statistical analysis
Categorical data were summarized and reported as frequencies and percentages and were compared using chi-squared test or Fisher’s exact test, as appropriate. Continuous variables were summarized and reported as means±standard deviation or medians and interquartile range; they were compared using Student’s t test or the Mann-Whitney U test, depending on whether the variables had a normal distribution. Survival curves were created using the Kaplan-Meier method and compared using the log-rank test. Factors associated with TTR and OS were identified using univariable and multivariable Cox proportional hazards regression models. All variables significant (P<0.05) in univariable analysis were entered into step-down Cox proportional hazards regression analysis. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated for all the regression results. All the analyses were performed using SPSS version 23 (IBM Corporation, Armonk, NY, USA). Propensity score analysis and matching were used with the psmatching program. All analyses were performed in R 3.3.3 software (R Foundation for Statistical Computing, Vienna, Austria). P values <0.05 considered statistically significant.
RESULTS
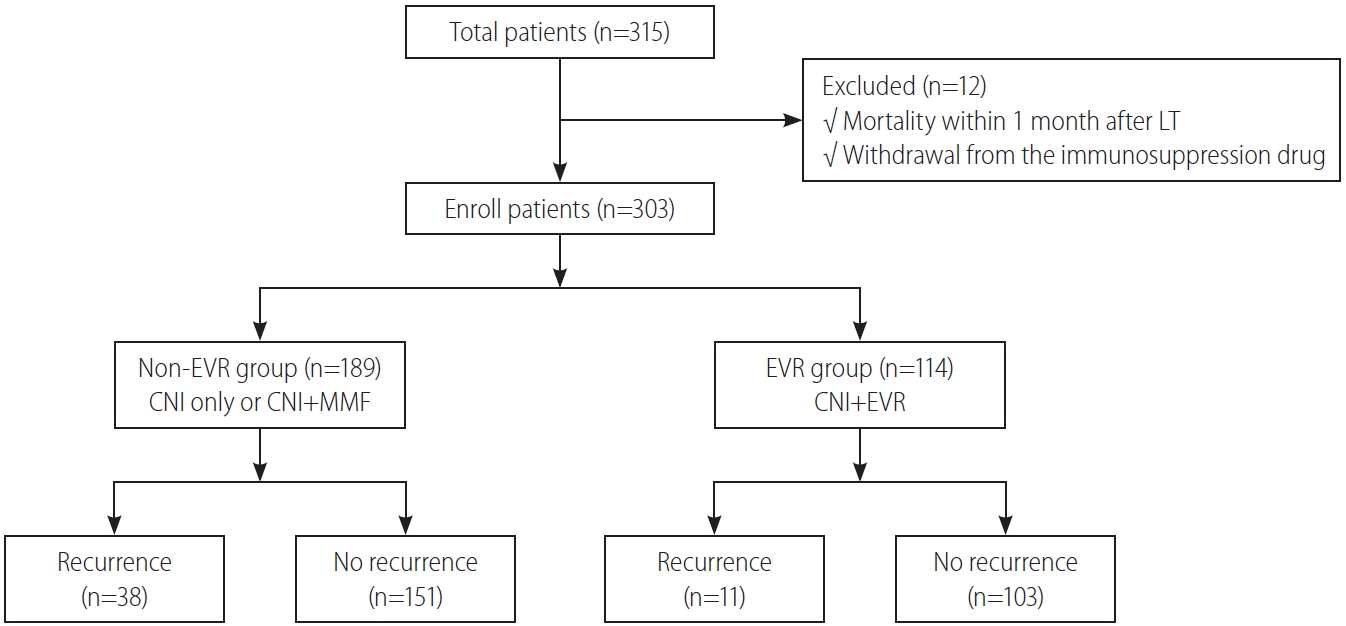
From January 2012 to July 2018, 315 patients underwent LT for HCC at Severance Hospital. We excluded 12 patients who met the aforementioned exclusion criteria. Among the 303 patients remaining in the study, 114 (37.6%) were included in the EVR group and 189 (62.4%) were included in the non-EVR group.
Baseline characteristics
Table 1 shows the baseline characteristics of the 303 patients included in the study. Of these, 247 (81.5%) were men and 56 (18.5%) were women, with a median age of 56 years (range, 34– 73). All the patients had histologically confirmed HCC. Overall, 238 patients (78.5%) were positive for hepatitis B virus infection, and 24 (7.9%) were positive for hepatitis C virus infection. The mean maximum tumor size was 2.5 cm, and 47 patients (15.5%) had undergone ABO-incompatible LT. Living donor LT was performed in 216 patients (71.3%), whereas 87 patients (28.7%) received deceased donor grafts. Tumors exceeded the Milan criteria in 77 patients (25.4%). The median follow-up period was 37.1 months (interquartile range, 1.2–80.2).
Clinicopathologic characteristics according to EVR usage
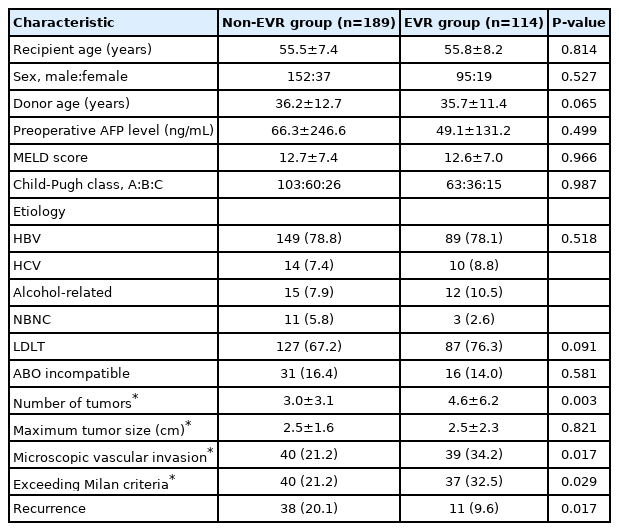
The clinicopathologic characteristics of patients in the non-EVR and EVR groups are shown in Table 2. Compared with non-EVR group, the EVR group had a significantly lower donor age (P=0.021) and a higher percentage of patients with MiVI (P=0.017) or exceeding the Milan criteria (P=0.029). Patients in the EVR group also had a higher number of tumors than those in the non-EVR group (P=0.003). Other clinicopathologic parameters did not differ significantly between the EVR and non-EVR groups.
Actual drug exposure
The mean trough tacrolimus levels within the first months after LT were not significantly different between the groups (non-EVR: 10.56±2.15 ng/mL; EVR: 10.97±2.28 ng/mL; at 7 days P=0.876). However, the introduction of EVR was followed by a stepwise decrease in tacrolimus exposure in the EVR group. The mean trough tacrolimus level was reduced from 10.96±3.67 ng/mL at initial to 3.15±2.31 ng/mL at 24 months. By contrast, the mean trough tacrolimus level in the non-EVR group remained at approximately 6–7 ng/mL from 4 months onward. Thus, the trough tacrolimus concentrations remained significantly reduced in the EVR group during the follow-up period compared with those in the non-EVR group (Supplementary Fig. 1A).
The mean trough level of EVR at 1 month post-LT was 2.12±1.23 ng/mL but increased and persisted within the target range (3–5 ng/mL) from 2 months onward: 3.05±1.38 ng/mL at 2 months, 4.16±1.45 ng/mL at 4 months, 3.89±2.36 ng/mL at 6 months, 4.35±1.84 ng/mL at 8 months, 3.82±1.69 ng/mL at 10 months, 3.32±1.96 ng/mL at 12 months, and 3.45±1.78 ng/mL at 24 months (Supplementary Fig. 1B).
Survival differences between the non-EVR and EVR groups
During the median follow-up of 37.1 months, 49 patients (16.2%) developed tumor recurrence and 53 (17.5%) died. When examining the entire cohort, the 1-, 3-, and 5-year TTR rates were 90.1%, 81.5%, and 80.0%, respectively, while the 1-, 3-, and 5-year OS rates were 91.2%, 82.4%, and 77.7%, respectively. The TTR curves for the EVR and non-EVR groups are shown in Figure 3A. TTR was significantly longer in the EVR group than in the Non-EVR group (P=0.029). The OS curves of patients according to EVR are shown in Figure 3B. Similar to TTR, OS was significantly longer in the EVR group (P<0.001).
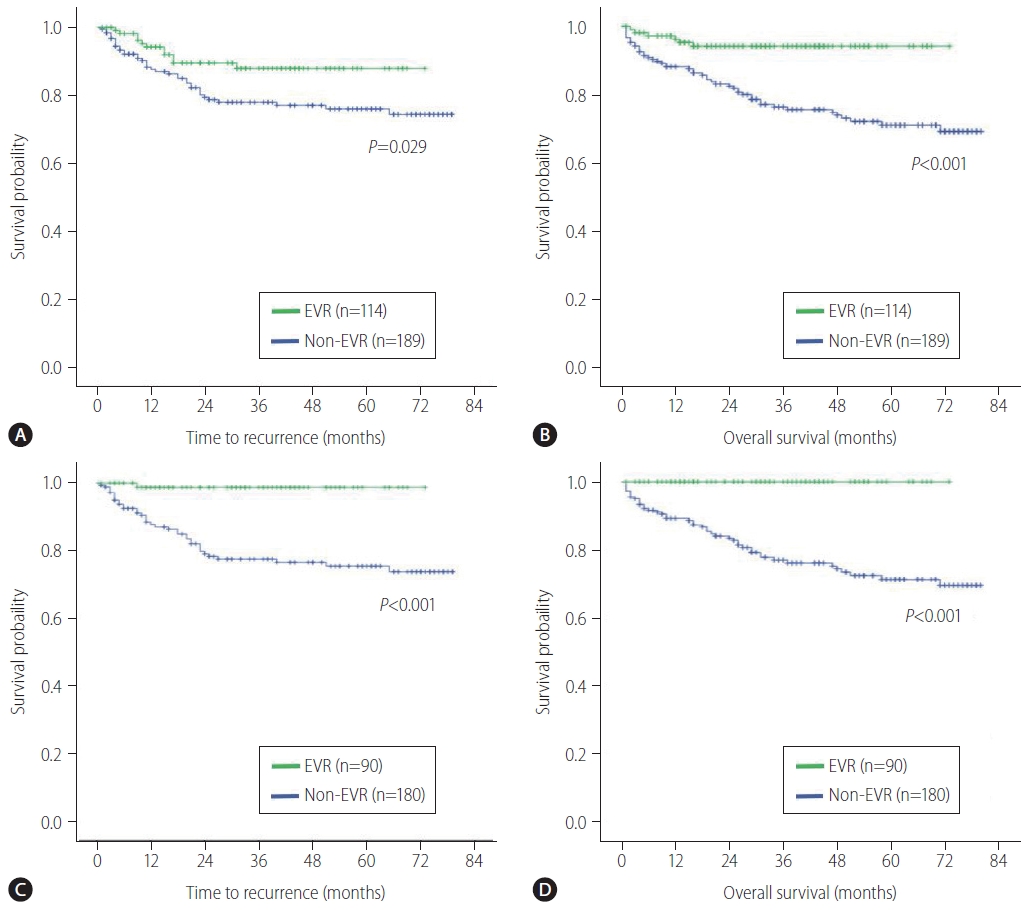
Kaplan-Meier survival curves of patients who had undergone liver transplantation for hepatocellular carcinoma. Time to recurrence (A) and overall survival (B) according to EVR use before propensity score matching. Time to recurrence (C) and overall survival (D) according to EVR after propensity score matching. EVR, everolimus.
Propensity score-matched comparison of non-EVR and EVR groups
Since this study was retrospectively designed in the first place, there were no consistent criteria for the selection of EVR over time. To minimize the selection bias and balance the differences in the baseline covariates between the non-EVR and EVR groups, we conducted propensity score matching (PSM) analysis. TTR and OS were compared between the two groups utilizing PSM.
After the 1:2 matching, 270 patients were selected and divided into propensity-matched EVR group (n=90) and propensity-matched non-EVR group (n=180). Table 3 summarizes the clinicopathologic characteristics between the two groups after PSM. The baseline variables were well balanced, and there were no significant clinicopathologic characteristics between the groups. In the matched population, cases with EVR had better TTR and OS (P<0.001) in comparison with cases in the non-EVR cohort (Fig. 3C, D). The result after PSM confirmed that the difference in survival rates between the groups was more remarkable than that before PSM.
Subgroup analysis of survival stratified by MiVI and Milan criteria
To evaluate the effects of EVR in low-risk versus high-risk patients, we performed subgroup analysis based on MiVI status (Supplementary Fig. 2) and Milan criteria (Supplementary Fig. 3), respectively. Low-risk patients were defined as patients without MiVI or within Milan criteria, and high-risk patients were defined as patients with MiVI or above Milan criteria.
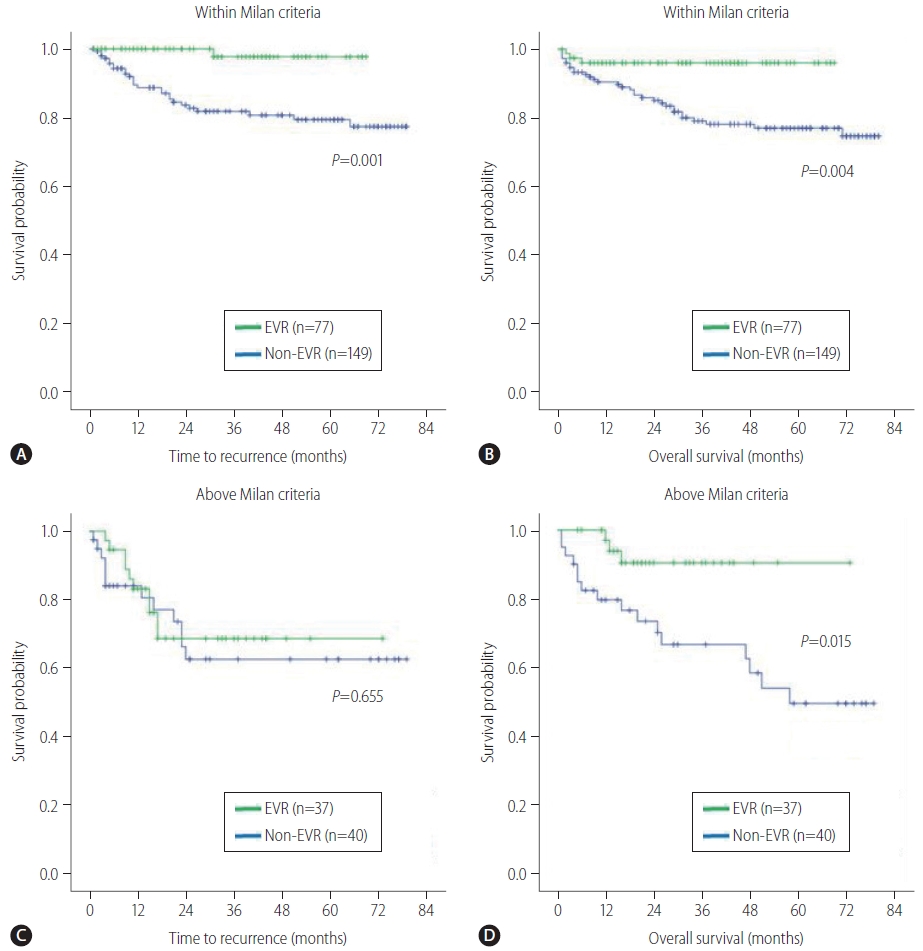
In patients without MiVI (n=223), the EVR group had substantially better TTR (P=0.005) and OS (P=0.001) than the non-EVR group (Fig. 4A, B). In patients with MiVI (n=80), the two groups had similar TTR (P=0.069; Fig. 4C), but the EVR group had a significantly longer OS (P=0.015; Fig. 4D). In patients within Milan criteria (n=226), the EVR group had substantially better TTR (P=0.001) and OS (P=0.004) than the non-EVR group (Fig. 5A, B). In patients above Milan criteria (n=77), no statistically significant difference in TTR (P=0.655; Fig. 5C) was found between the EVR and non-EVR groups, but the EVR group had significantly longer OS (P=0.015; Fig. 5D).

Subgroup analysis according to the presence of MiVI. Time to recurrence (A) and overall survival (B) according to EVR use in patients without MiVI. Time to recurrence (C) and overall survival (D) according to EVR use in patients with MiVI. MiVI, microscopic vascular invasion; EVR, everolimus.
Posttransplant complications
Surgical and immunological complications for the two groups are listed in Table 4. During the follow-up period, 25 cases (13.2%) and 15 cases (13.1%) of suspected acute cellular rejection were observed in the non-EVR and EVR groups, respectively. All patients with acute cellular rejection were treated with steroid pulse therapy. Three cases in the non-EVR group developed hepatic artery thrombosis (HAT) versus none in the EVR group. There were no statistically significant differences between the EVR and non-EVR groups in the occurrence rate of suspected acute cellular rejection; biliary complications, including biliary stricture and leak; portal vein thrombosis; wound infection; mouth ulcers; and renal failure. Dyslipidemia (P=0.009) and proteinuria (P=0.008) were significantly more frequent adverse effects in the EVR group than in the non-EVR group.
Prognostic factors for time to recurrence
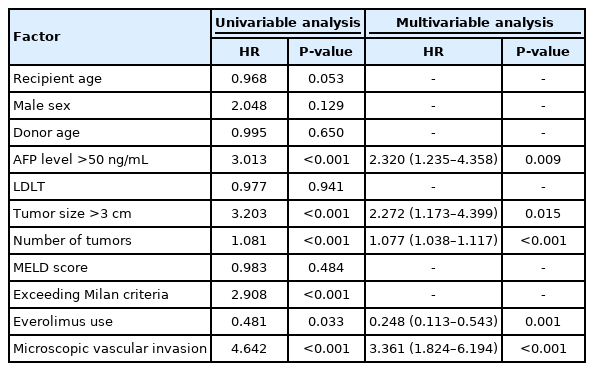
On univariable analysis, prognostic factors significantly associated with a worse TTR were an AFP level >50 ng/mL, tumor size >3 cm, higher number of tumors, exceeding Milan criteria, EVR use, and the presence of MiVI. In multivariable analysis, the following were identified as independent prognostic factors for TTR: AFP >50 ng/mL (HR, 2.320; 95% CI, 1.235–4.358; P=0.009), tumor size >3 cm (HR, 2.272; 95% CI, 1.173–4.399; P=0.015), a higher number of tumors (HR, 1.077; 95% CI, 1.038–1.117; P<0.001), the presence of MiVI (HR, 3.361; 95% CI, 1.824–6.194; P<0.001), and EVR use (HR, 0.248; 95% CI, 0.113–0.543; P=0.001) (Table 5). Exceeding Milan criteria was excluded from the multivariable analysis because it was designated as an overlapping factor with tumor size and tumor numbers.
Prognostic factors for overall survival
Univariable analysis showed that an AFP level >50 ng/mL, tumor size >3 cm, a higher number of tumors, a higher MELD score, exceeding Milan Criteria, the presence of MiVI, and EVR use affected OS. In multivariable analysis, the following were identified as independent prognostic factors for OS: an AFP level >50 ng/mL (HR, 2.120; 95% CI, 1.127–3.987; P=0.020), a higher number of tumors (HR, 1.059; 95% CI, 1.013–1.106; P=0.011), a higher MELD score (HR, 1.044; 95% CI, 1.007–1.083; P=0.019), the presence of MiVI (HR, 1.976; 95% CI, 1.073–3.640; P=0.029), and EVR use (HR, 0.145; 95% CI, 0.055–0.381; P<0.001) (Table 6).
DISCUSSION
Despite major advances in transplant immunosuppressant regimens in recent decades, the ideal immunosuppression protocol for liver transplant recipients with HCC remains debatable. The primary goal of immunosuppression treatment in transplantation is to maintain a balance between the risk of graft rejection and risk of immunosuppression-induced malignancy. Therefore, selecting the optimal immunosuppressant regimen is crucial for improving survival after LT for HCC.
Over the past decade, CNIs (tacrolimus and cyclosporin) have remained the primary maintenance immunosuppressants after LT [15]. However, numerous studies have reported CNIs (particularly tacrolimus) as a dose-dependent factor for increasing the risk of tumor recurrence after LT for HCC [12,13,16,17]. Furthermore, reducing the tacrolimus dosage by adding EVR has been demonstrated to improve post-transplant renal function compared with CNI alone [18,19]. Therefore, these results suggest that minimizing the CNI levels should be considered to reduce the risks of HCC recurrence and renal toxicity.
The anti-proliferative and anti-tumor effects of mTORi (sirolimus and EVR), which were introduced as alternative immunosuppressant agents to CNI, have been suggested by previous animal and retrospective studies [20-22]. Among mTORi, EVR was developed more recently and has more potent anti-proliferation effects than sirolimus [23,24]. Klawitter et al. [24] highlighted the substantially different pharmacodynamic and toxicodynamic properties between sirolimus and EVR. For these reasons, we have incorporated EVR into our post-LT protocol for patients with HCC to optimize the immunosuppressant regimen.
Regarding the timing of EVR use, we initiate EVR in combination with the CNI 4 weeks after LT, representing the same protocol used in the H2304/H2307 trials involving LT [18,25]. This initiation time was chosen because of previously reported concerns of irreversible kidney damage [18,26], wound healing [27,28], and HAT [29]. No EVR-related wound healing problems or HAT was detected in any of our 114 patients who received EVR.
In the absence of guidelines for EVR usage, previous studies have attempted to use EVR in various ways, including early or late initiation times, as monotherapy or combination therapy, and with a wide spectrum of target blood trough levels. Because of these heterogeneous conditions, the outcomes associated with EVR usage have varied considerably. However, in the present study, we have been following a unified immunosuppressive protocol since 2012. Based on our findings, we believe that our protocol may provide clinically useful guidelines for using EVR.
In the present study, the EVR group had more aggressive tumor biology than the non-EVR group, as reflected by a higher number of tumors and a higher prevalence of MiVI and cases exceeding Milan criteria. These factors can be used to identify patients at a high risk of recurrence after surgery. Unexpectedly, the EVR group had better TTR and OS than the non-EVR group, despite the EVR group having patients with more advanced tumor characteristics. The result after PSM showed a more dramatic improvement in the survival rate of the EVR group compared to the non-EVR group. MiVI has been identified as one of the strongest risk factors for poor outcomes after LT [11,30]. Lim et al. [10] argued that MiVI is a better predictor of tumor recurrence and OS in HCC than Milan criteria. However, MiVI can only be detected by pathologic examination after LT, and no evidence currently indicates that postoperative adjuvant therapy provides a survival benefit after LT for HCC in patients with pathologically confirmed MiVI.
In subgroup analysis of high-risk patients with MiVI or above Milan criteria, EVR use was associated with improved OS but not TTR. This discrepancy between OS and TTR was unexpected. One possibility is that EVR has no direct impact on tumor recurrence in high-risk patients. As with advanced HCC, tumor recurrence is more likely influenced by aggressive factors related to the tumor itself. However, the anti-tumor effects of EVR may slow the rate of tumor growth and progression after recurrence, contributing to improved OS. This result may be a crucial clue suggesting that EVR may be useful not only as an immunosuppressant agent but also as an adjuvant therapeutic agent.
In low-risk patients, EVR was clearly beneficial for both tumor recurrence and survival. Only one recurrence was noted among the 75 MiVI-negative or 77 within-Milan criteria patients who received EVR. These results are consistent with the findings of the recent multicenter randomized SILVER trial, which showed that sirolimus was associated with improved disease-free survival in the first 3–5 years after LT, particularly in low-risk patients [31]. Our results suggest that the anti-cancer effects of EVR are more pronounced in low-risk patients than in high-risk patients and that immunosuppressant protocols should be tailored to individual risk stratification based on tumor biology. A well-designed prospective study is required to more definitively determine the role of EVR for HCC.
In the present study, EVR use was an independent significant prognostic factor for improved TTR and OS after LT for HCC. Until now, the anti-tumor effects in HCC patients have been considered to reflect reduced CNI exposure rather than direct EVR effects. To our best knowledge, this is the first report describing the independent prognostic value of EVR use in patients with HCC after LT. To decrease the risk of bias, multiple parameters that could potentially impact survival were adjusted for. Unexpectedly, after correction for multiple variables, EVR gained more statistical power as an independent prognostic factor in multivariable analysis. A preoperative AFP level >50 ng/mL and the presence of MiVI were also strong indicators of prognosis. These results are consistent with the findings of previous studies [11].
The current study had some limitations. The data for this retrospective study were extracted from medical records, and the study was conducted at a single institution. PSM analysis was used to reduce the selection bias and confounding variables between the groups. However, there are some selection biases due to the retrospective design of the study. Based on our findings, a well-designed randomized controlled trial is required to establish the role of EVR for HCC. Despite these limitations, we showed potential survival benefits of EVR-based immunosuppressant therapy, even in patients with MiVI. Our findings provide a better understanding of the anti-cancer effects of EVR and may be helpful in selecting post-LT immunosuppressant agents in other patients with HCC.
In conclusion, an immunosuppression protocol comprising EVR combined with lower dose CNIs was associated with improved long-term survival after LT for HCC. EVR was particularly beneficial for reducing tumor recurrence and improving survival in lowrisk patients. EVR use was identified as an independent significant prognostic factor for improved TTR and OS after LT in patients with HCC. These results provide useful information to improve the management of liver transplant recipients with HCC. Further well-designed prospective studies should be conducted to confirm our results.
Notes
Authors’ contributions
Incheon Kang: study design, statistical analysis, writing and revision of the manuscript; Hyun Jeong Kim: data acquisition, statistical analysis, data interpretation; Jae Geun Lee, Sung Hoon Choi, Dai Hoon Han, Gi Hong Choi, Myoung Soo Kim, Jin Sub Choi, and Soon Il Kim: study design, data interpretation, revision of the manuscript; Dong Jin Joo: study design, data interpretation, writing and revision of the manuscript.
Conflicts of Interest
The authors have no conflicts to disclose.
SUPPLEMENTAL MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
Mean trough levels for tacrolimus (A) and everolimus (EVR) (B) during the follow-up period. The values are shown as means. The shaded areas indicate target ranges.
Study flow diagram for subgroup analysis stratified by microscopic vascular invasion. MiVI, microscopic vascular invasion; EVR, everolimus.
Study flow diagram for subgroup analysis stratified by MC. MC, Milan criteria; EVR, everolimus.
Abbreviations
AFP
alpha-fetoprotein
CI
confidence interval
CNI
calcineurin inhibitor
EVR
everolimus
HAT
hepatic artery thrombosis
HCC
hepatocellular carcinoma
HR
hazard ratio
LT
liver transplantation
MELD
model for end-stage liver disease
MiVI
microscopic vascular invasion
mTORi
mammalian target of rapamycin inhibitor
OS
overall survival
PSM
propensity score matching
TTR
time to recurrence
References
Article information Continued
Notes
Study Highlights
We reviewed data from 303 consecutive patients with hepatocellular carcinoma who underwent liver transplantation. After transplantation, patients received a CNI plus everolimus or a CNI without everolimus. Although patients who received a CNI plus everolimus had more aggressive tumor biology, they had better time to recurrence and overall survival than those who received a CNI without everolimus. On multivariable analysis, use of everolimus was identified as an independent prognostic factor for improved time to recurrence and overall survival.