| Clin Mol Hepatol > Volume 28(3); 2022 > Article |
|
ABSTRACT
Treatment of intrahepatic cholangiocarcinoma (iCCA) is currently at a significant turning point due to the identification of isocitrate dehydrogenase (IDH) mutations and fibroblast growth factor receptor (FGFR) fusions that can be targeted with currently available therapies. Clinical trials of these targeted therapies have been promising, and the iCCA patients who may benefit from these targeted treatments can be identified by pathological examination prior to molecular investigations. This is because IDH mutations and FGFR fusions are mainly seen in the small duct type iCCA, a subtype of iCCA defined by the 5th World Health Organization classification, which can be recognized by the pathological diagnostic process. Therefore, pathology plays an important role in precision medicine for iCCA, not only in confirming the diagnosis, but also in identifying the iCCA patients who may benefit from targeted treatments. However, caution is advised with the pathological diagnosis, as iCCA shows tumour heterogeneity, making it difficult to distinguish small duct type iCCA from hepatocellular carcinoma (HCC), and combined HCC-CCA. This review focuses on the pathological/molecular features of both subtypes of iCCA (large and small duct types), as well as their diagnostic pitfalls, clinical relevance, and future perspectives.
Cholangiocarcinoma (CCA) is anatomically categorized into three groups: intrahepatic CCA (iCCA), arising from the periphery of the second-order bile ducts; perihilar CCA (pCCA), located at the right, and/or left, hepatic duct, and/or at their junction; and distal CCA (dCCA), involving the common bile duct [1,2]. iCCA is further subcategorized into the large duct type and the small duct type, according to the 5th World Health Organization (WHO) classification [1]. Importantly, the clinicopathological and molecular features of the small duct type iCCA are quite different from those of the large duct type iCCA, whose patho-molecular features are, in fact, more similar to that of pCCA, or dCCA [1-3]. In addition, fibroblast growth factor receptor (FGFR) fusion is observed in around 5.7–14% [4-6] of iCCA cases, and isocitrate dehydrogenase (IDH)-1 and -2 mutations are identified in around 20–30% [5,6]. These actionable mutations are mainly seen in the iCCA small duct type. Clinical trials for targeted therapy of FGFR2 and IDH1 have shown very promising results [7-9]. In brief, the final overall survival (OS) efficacy results of ivosidenib, an IDH1 inhibitor, in advanced iCCA patients with IDH1 mutations, showed a favourable OS compared to placebo [8]. Infigratinib, an FGFR2 inhibitor, presented promising clinical activity and a manageable adverse event profile in patients with histologically/ cytologically confirmed, and previously treated, advanced, or metastatic CCA, harbouring FGFR2 gene fusions or rearrangements [9]. In addition, FGFR2 and IDH1 inhibitors have been approved and are beginning to be used in iCCA patients. This is very welcome news as iCCA is a more aggressive and lethal cancer compared to hepatocellular carcinoma (HCC), largely as a result of the delays in diagnosis due to the lack of an adequate surveillance system and a high post-operative recurrence rate, even after curative-intent surgical resection [10,11]. Moreover, unlike HCC, liver transplantation is an option in only very select cases of iCCA [12]. Finally, iCCA mortality is increasing worldwide with a rise in incidence [13], and the efficacy of the current chemotherapy is limited. Therefore, an accurate diagnosis of iCCA and appropriate classification are essential to allow for effective precision medicine.
However, an iCCA diagnosis is not always straightforward. Pathologically, iCCA, especially the small duct type, is well-known to show tumour heterogeneity, which correlates with radiological heterogeneity, which makes it difficult to diagnose by imaging [14]. Moreover, the small duct type iCCA shares similar clinical features with those of HCC, namely a mass-forming tumour, and frequent association with chronic liver disease. Therefore, it is essential for pathologists and clinicians to understand iCCA tumour heterogeneity, including its differential diagnosis, to make a proper diagnosis.
With these factors in mind, this comprehensive review focuses on the updates of iCCA subtypes and their clinical and genetic relevance, based on the latest WHO classification and consensus papers. Diagnostic pitfalls are also covered, which will assist clinicians and pathologists to link pathological features with clinical relevance.
As cancer mimics its normal counterpart, it is very important to understand its cellular origin. iCCA arises from cholangiocytes, which are the lining epithelia of the biliary tracts. The bile duct presents as a three-dimensional tree-like structure, and the size of the bile duct becomes smaller towards its periphery. Interestingly, the phenotype of cholangiocytes, in terms of size and characteristics, differs depending on their anatomical location. For instance, the large bile duct is lined by mucin-producing cylindrical cholangiocytes, while the small duct is lined by mucin-negative cuboidal cholangiocytes [15]. These phenotype differences are also evident in the immunohistochemistry. Epithelial membrane antigen (EMA) staining shows strong cytoplasmic positivity in the mucin-producing cylindrical cholangiocytes in the large bile duct, whereas the mucin-negative cuboidal cholangiocytes located in the small bile duct exhibit an apical expression pattern [15]. Therefore, it is understandable that the large bile duct gives rise to the mucin-producing iCCA with EMA cytoplasmic expression, and the small duct type leads to the mucinnegative iCCA with apical EMA positivity. The 5th WHO classification uses this theory to subclassify iCCA into two groups, the small duct type and the large duct type [1].
Large duct type iCCA is mainly located in the proximity of the hepatic hilum area. Macroscopically, large duct type iCCA mainly presents with a periductal infiltrating (PI) pattern, and PI with mass forming (MF) pattern. The PI pattern is caused by the thickening of the large bile duct wall due to cancer infiltration, associated with perineural invasion and/or lymphatic invasion. Intrahepatic bile duct in the periphery of the tumour shows dilatation due to the proximal bile duct stricture. Mixed PI and MF type is an iCCA tumour which shows tumour infiltrates along the bile duct (PI pattern), with concurrent invasion into neighbouring liver parenchyma, leading to the MF. Since the small duct type iCCA presents with MF pattern only, identification of the PI component is essential to distinguish PI+MF (large duct type) from MF only (small duct type). The frequency of the macroscopic pattern is 9.7% for PI type and 12.3% for the mixed PI and MF pattern [16].
Histologically, large duct type iCCA exhibits a clear glandular structure with mucin production associated with desmoplastic reaction and inflammatory cell infiltration [15]. Desmoplastic reaction in the large duct type iCCA is more solid and hyalinized compared to that in the small duct type iCCA, and entrapped portal tracts are usually not seen in this subtype. Importantly, the large bile duct type iCCA shows aggressive pathological features, such as lymphatic invasion and perineural invasion. Also, the pathological features of the large duct iCCA show similarities to those of hilar and extrahepatic CCA and pancreatic cancer, as a reflection of their similar embryonal origin.
Clinically, chronic biliary inflammations, such as primary sclerosing cholangitis, hepatolithiasis, and liver fluke infection, are known to be risk factors for large duct type iCCA [1]. In addition, biliary intraepithelial neoplasia and intraductal papillary neoplasm are known to be precursor lesions of large duct type ICC [1,17]. Molecular profiles showing KRAS, TP53, and cyclin-dependent kinase inhibitor 2A (CDKN2A) alterations are often seen in the large duct type iCCA [3]. As with the histological features, the molecular features of the large duct type iCCA have shown some similarities with those of pCCA, dCCA, and pancreatic cancers. The molecular profiles of the large duct iCCA will be discussed in more detail in another chapter.
Small duct type iCCA is mainly located in the periphery of the liver. However, around 30% of the small duct type iCCA is observed in the perihilar area [15]. The most important feature of the small duct type iCCA is that it always presents as a MF, which is the biggest point of difference from the large duct type iCCA. In addition, the co-existence of chronic liver diseases, such as chronic viral hepatitis and steatohepatitis, is quite common in this type of tumour [1].
The tumour shows an expansive growth pattern without fibrous capsule, which sometimes resembles normal liver parenchyma. The entrapped normal portal tracts are often seen inside the tumour. Pathologically, it is characterized as a heterogeneous tumour due to a varying pattern of ductular proliferation, such as irregular glandular structure mimicking ductular reaction-like features, small uniform glandular proliferation, and a narrowing ductal lumen with anastomosing glandular features. The tumour associates with fibrous stroma, which is often oedematous and exhibits fine fibrosis with prominent inflammation compared to that of the large duct type iCCA. The tumour cells show no significant cellular atypia or pleomorphism and are described as having an “innocent-looking” aspect; one of the characteristics of the small duct type iCCA. Mucin staining confirms the absence of mucin production of the small duct type iCCA. EMA staining shows an apical expression pattern with or without some degree of weak cytoplasmic expression. The absence of mucin production and apical EMA staining can be very helpful to distinguish the small duct type iCCA from the large duct type iCCA [15]. Compared with the large duct type iCCA, perineural invasion or lymphatic invasion, are usually less common in the small duct type iCCA, although these features may become more frequent in larger-sized small duct type iCCA. At the tumour/non-tumour border, the tumour cells show a type of replacing growth pattern. Background liver shows a chronic hepatitis with some degrees of fibrosis.
Clinically, chronic viral hepatitis, non-biliary cirrhosis, and steatohepatitis are commonly seen in this type of tumour [1]. Unlike the large duct type iCCA, precursor lesions are unknown for the small type iCCA. An important feature is that actionable mutations, such as IDH mutations and FGFR2 fusions, are only seen in this type of tumour. This will be discussed in the next chapter.
Finally, the prognosis of the small duct type iCCA is much better than that of the large duct type iCCA [17-19]. In summary, iCCA comprises two very distinct subtypes, the small duct type and the large duct type. This review illustrates the importance of distinguishing between the two subtypes, as well as the significance of their pathological diagnosis, and hence the validity of this classification.
Several studies have demonstrated a clear correlation between clinicopathological features and genetic findings [17,19,20]. Importantly, the iCCA categorization used in these studies correlates with the WHO classification. For instance, unsupervised clustering categorization, subclass A (hepatitis aetiology, better prognosis, cholangiolar-type pathology, IDH, and FGFR genetic alterations) corresponds to the small duct type iCCA, and subclass B (cholangitis aetiology, worse prognosis, bile-duct type pathology, Kras mutation) is consistent with the large duct type iCCA [20]. In another study, where cases were categorized as either bile duct type (Kras mutation) or cholangiolar type (IDH1/2 mutation) [19], and a further study where they were categorized as either perihilar type (SMAD4 loss, worse prognosis) or peripheral type (viral hepatitis aetiology, IDH1 mutation, BRCA associated protein 1 [BAP1] loss) [17], both categories in each study corresponded to the large and small duct type iCCA, respectively (Tables 1, 2) [1].
Other studies have used different approaches to characterise the molecular basis of iCCA, such as tumour location (intra- vs. extra-hepatic) [3], aetiology [21], prognosis [22-24], sample type (resection vs. biopsy) [25], and integrative genomic analysis [26]. There results also present some similarities with the iCCA subtype, as defined by the WHO classification. For example, a study examining the frequency of genetic alterations in intra- and extra-hepatic CCA (eCCA; pCCA and dCCA) showed that iCCA has some specific genetic alterations, i.e., IDH1, FGFR2, and BAP1, as does eCCA, i.e., SMAD4 and ERBB2 [3]. Interestingly, both iCCA and eCCA showed TP53, CDKN2A, and KRAS mutations, likely due to the similarity between the large duct type iCCA and eCCA; both originating from the large bile duct. In terms of aetiology, mostly fluke-positive groups showed TP53 and ERBB2 (also called HER2) mutations [21]. This data corresponds to that of the large duct type iCCA, as fluke infections give rise to chronic inflammation in the large bile duct, which can lead to large duct type iCCA. In contrast, mostly fluke-negative and iCCA groups demonstrated FGFR, BAP1, and IDH1/2 mutations, which are more representative of the small duct type iCCA.
In terms of the prognosis, TP53, KRAS, and CDKN2A alterations have been shown to be predictive of worse OS after surgery [22-24]. This is indicative of the large duct type iCCA, which often harbours these mutations, as it shows more aggressive pathology and a poor prognosis compared to the small duct type iCCA. The IDH mutant subtype shows distinct molecular features as well as an expanded histological spectrum, which probably corresponds to the small duct type iCCA [26].
A challenging aspect of molecular profiling in cancer is that molecular alterations are known to be different between early and advanced cancer stages. Interestingly, however, the early (resected) and advanced stages (non-resected cases) of iCCA present with quite similar profiles [22]. This will be an advantage for iCCA molecular profiling, as surgical specimens obtained from early-stage patients contain enough tumour tissues for the molecular investigation. In addition, this may make it possible to investigate the correlation between molecular alterations and histological variations.
Finally, it is important to mention the incidence of molecular alterations relative to the type and location of the tumour sampling (i.e., primary, metastatic, and liquid biopsy) [25]. For instance, the IDH1 mutation is identified at a rate of 16% in primary tumour biopsies, but its incidence is much lower in metastatic sites (5%) and liquid biopsies (9%). Similar phenomena have been observed with FGFR2. Therefore, it is necessary to consider which method will be used for the molecular test.
In summary, molecular profiling appears to have a clear correlation with the iCCA subtypes, such as the small and large duct types. This confirms the utility of this classification as well as the importance of a pathological assessment of iCCA.
In the liver, there are three types of MF primary liver tumours arising in chronic liver diseases, namely HCC, combined HCC (cHCC)-CCA, and small duct type iCCA. These tumours show a continuous histological spectrum, making it difficult to diagnose them properly [14]. As mentioned in the previous chapters, a proper diagnosis of iCCA, including subtypes, is essential, as it directly relates to the treatment choice. Therefore, one should not hesitate to ask an experienced liver pathologist for a second opinion when dealing with a challenging liver tumour diagnosis.
CLC was first described by Steiner and Higginson as a unique primary liver cancer presenting with small ductules resembling “cholangioles.” [27] CLC forms a mass, frequently associated with chronic liver disease, and is identified as a hypervascular tumour by imaging [28]. Due to these features, CLC is at risk of being clinically diagnosed as HCC. However, the pathological features of CLC are quite different from those of HCC; CLC comprises a ductular-reaction like tumour structure in more than 80% of the tumour area [1]. In addition, CLC may have a CCA area and/or a hepatocytic differentiation area within the tumour [28]. Therefore, the 5th WHO classification categorizes CLC differently based on the presence of hepatocytic differentiation [1,29], i.e., CLC is defined as cHCC-CCA if the tumour contains hepatocytic differentiation, and the remaining CLC is categorized into small duct type iCCA (Fig. 3). This categorisation is made for two reasons. Firstly, the molecular data from CLC without hepatocytic differentiation shows biliary phenotypes which have a different genomic status compared with cHCC-CCA [30]. In addition, cHCC-CCA is a tumour which comprises both hepatocytic and cholangiocytic differentiation; therefore, to consolidate the definition of cHCCCCA, CLC must have a hepatocytic differentiation to be categorized as cHCC-CCA. As such, it is very reasonable and understandable that the 5th WHO classification has made the recategorization [1,29]; however, CLC has a distinct feature in terms of its clinico-radiological presentation where it mimics HCC, and usually has a better prognosis [28]. Therefore, further investigation is required to evaluate the nature of CLC to assess how to categorize this tumour.
As indicated by the name, cHCC-CCA is a tumour comprising both hepatocytic and cholangiocytic differentiation [31]. The HCC component shows a trabecular growth pattern composed of tumour cells with eosinophilic cytoplasm, and round nuclei without mucin production. In contrast, the CCA area shows a glandular structure with mucin production and is associated with fibrous stroma. These different tumour components show transitional features, in contrast to collision tumours that contain separate HCC and CCA without transitional features. The problem is that the diagnosis is made regardless of the percentage of each component [29], making it challenging to diagnose by both pathology and imaging. Moreover, a typical cHCC-CCA, where both components can be easily recognised by hematoxylin and eosin staining, comprises only 18% of total cases [32], and evaluating the origin of the remaining cases presents a unique challenge due to tumour heterogeneity. For instance, a tumour may show histological diversity comprising different components of ductular configurations, solid pattern, and glandular structure [31]. Another problem is the lack of a clear definition of hepatocytic differentiation. Currently, we use immunohistochemistry to evaluate hepatocytic differentiation, but there are several antibodies available and their sensitivity varies. Therefore, one may anticipate that the identification of hepatocytic differentiation may differ depending on the immunohistochemical antibody used.
Finally, the percentage of HCC and iCCA components in cHCC-CCA is different for each case, which makes it difficult to diagnose by imaging [33]. For example, in cHCC-CCA with predominant HCC component, the radiological features are similar to those of HCC, while cHCC-CCA with predominant CCA component appears similar to iCCA on imaging [33]. Liver image in reporting and data system (Li-RADS) uses the standardisation of images in assessments [34]. According to this system, LR4 is probably HCC, and LR5 is considered a definite HCC. However, a Korean group reported that 11.9% of cHCCCCA was assigned to LR4, and 35.5% of cHCC-CCA was assigned to LR5 [35]. This data highlights the difficulty of diagnosing cHCC-CCA by imaging. Similar issues can be expected with tumour biopsy. However, a renowned French group reported that a combination of imaging and tumour biopsy examination improved the diagnostic sensitivity compared to imaging or pathological investigation alone [36]. This data indicates that the use of both modalities makes it possible to diagnose cHCC-CCA in a pre-surgical setting.
K19-positive HCC is a subtype of HCC that shows K19 expression in more than 5% of tumour cells [37]. K19-positive HCC is known to have a worse prognosis and earlier recurrence compared to K19-negative HCC [38]. Therefore, it is important to recognise this type of HCC. However, we must keep in mind that the K19 expression does not always mean cholangiocytic differentiation. The morphology of K19-positive HCC is the same as that of HCC, and there is no glandular structure indicating cholangiocytic differentiation. In addition, the K19 expression pattern is different from that of iCCA. Therefore, it is important to assess the nature of the tumour by morphology and immunohistochemistry.
The liver is a common place for metastatic adenocarcinoma, and therefore, it is important to distinguish between primary and metastatic adenocarcinoma before investigating the subtype of iCCA. The most challenging aspect to this is distinguishing between metastatic pancreatic cancer and primary iCCA, especially the large duct type. This is because both tumours show similar histochemical features, as well as immunohistochemical patterns. Since they require different treatments, the distinction should be made carefully and in consultation with a multidisciplinary team. Breast cancer liver metastasis also mimics the small duct type iCCA. Therefore, it is important that a clinical history is provided by the clinician to the pathologist to facilitate a more accurate diagnostic process.
ICCA needs to be distinguished from HCC and cHCC-CCA. This is not always straightforward, as these mass-forming primary liver tumours arising in chronic liver diseases show a continuous histological spectrum [14].
Several working groups, such as the National Comprehensive Cancer Network (NCCN), the European Society for Medical Oncology (ESMO), the British Society of Gastroenterology (BSG), and the International Liver Cancer Association (ILCA) have noted the importance of the utility of different modalities, such as serum tumour markers (serum CA19-9, carcinoembryonic antigen), imaging (magnetic resonance cholangiopancreatography), and tumour biopsy for ICC diagnostic workup [39]. Interestingly, tumour biopsy for potentially resectable lesions does not have full consensus. For instance, NCCN does not approve the utility of biopsy for this condition, whereas BSG and ILCA do recommend biopsy, even for potentially resectable lesions. The ESMO recommends a decision for the need of biopsy based on each patient’s situation. It is fully understandable why the guidelines are different, as a tumour biopsy still presents some risk. In contrast, there is no doubt of the necessity for tumour biopsy if a patient is heading for a systemic treatment regime. In fact, tumour biopsy presents several advantages; it will certainly improve the accuracy of diagnosis. In addition, the biopsy specimen can provide information on the tumour microenvironment, theranostic classification, and background liver condition. We would also particularly like to emphasise the fact that the assessment of non-tumoral tissue is important to perform systematic treatment safely. This is because around 20–30% of patients who show normal liver enzymes present with some pathological changes on the liver biopsy. In addition, steatohepatitis, which has become a common liver disease worldwide, is currently diagnosed only by liver biopsy [40]. We also need to keep in mind that a tumour biopsy can assess the tissue adequacy for the molecular test in terms of diagnosis and tumour components, such as cellularity, necrosis, fibrosis, and inflammation. Since the molecular test is an expensive tool, evaluating these points by tissue samples will certainly be beneficial for the patient as well.
It seems that 2 cm is an important cut-off value in terms of iCCA prognosis. Several studies have reported that the overall 5-year survival rate after curative resection is improved in cases where the iCCA tumour is smaller than 2 cm [16,41,42]. Indeed, very early iCCA (single tumours <2 cm) has shown a better prognosis; 5-year recurrence risk of 18%, and a survival rate of 65% in transplanted patients [42]. In general, the small duct type iCCA shows a better prognosis compared to the large duct type iCCA [17-19]. This is largely because the small duct type iCCA shows less perineural and lymphatic invasion compared to the large duct type iCCA. However, when the tumour becomes larger than 5 cm, even the small duct type iCCA starts presenting with aggressive pathological features, such as lymphatic and perineural invasions. Given that iCCA appears to become more aggressive when it reaches 2 cm, it may be interesting to investigate the molecular background to understand the carcinogenesis of ICCs sized less or more than 2 cm.
Up to 48% of iCCAs present with multiple tumours without other extrahepatic metastases [43]. Similar to HCC, there are two possibilities in terms of multiple iCCA, namely intrahepatic metastasis and de novo. A recent study reported that molecular alterations were concordant across all intrahepatic tumours in individual multiple iCCA patients [44]. This data, in some way, supports the proposal of modifying the iCCA staging system of the American Joint Committee on Cancer version 8, reported by the ILCA group [45]. They showed that the outcome was better stratified when multiple iCCA patients were categorised into the advanced group. In brief, multiple iCCA patients had a worse prognosis compared with stage I to III patients, and a better prognosis compared to patients with extrahepatic metastases [45]. However, from a pathological point of view, this data should be assessed carefully, since two distinct iCCAs were evaluated in the same group. Small duct iCCA occurs in chronic liver disease, which theoretically may have a higher possibility of harbouring de novo tumours compared to the large duct iCCA, although there have been no studies to illustrate this to date. In addition, tumour size is important for the prognosis, and further investigation is also recommended to clarify this point.
As in the case of other cancers, such as HCCs, immunotherapy is considered a potential treatment strategy in iCCA [46]. Immune checkpoint inhibitors (ICIs) act as immunotherapy agents by modulating immune checkpoint-mediated signalling pathways. Interestingly, the combination of ICIs with other, more standard therapies, especially chemotherapy, may be more effective compared to ICI monotherapy [46]. In fact, nivolumab (anti-programmed death-1 monoclonal antibody immunotherapy) with gemcitabine and cisplatin has shown promising results in phase II clinical trials in patients with non-resectable CCA [47,48]. As a result, the combination of gemcitabine and cisplatin with durvalumab versus gemcitabine and cisplatin with placebo, is currently being used in phase III clinical trials (NCT03875235).
The tumour micro-environment, especially the degree of inflammatory cell infiltration in the tumour, is well known to be a key factor in predicting the efficacy of immunotherapy. Sia et al. [23] reported that 38% of iCCAs were categorized as an inflammation class characterized as well differentiated tumour, having a better clinical outcome, and with activation of inflammatory signalling pathways. In comparison, iCCA subcategories, as defined by the WHO classification, have shown the small duct type iCCA to more commonly present with inflammation compared to the large duct type. Therefore, the efficacy of immunotherapy may be different in these iCCA types.
Since iCCA is a highly aggressive tumour, further investigation is urgently needed to expand future treatment choices for iCCA patients.
It is important to understand that iCCA comprises two distinct subtypes: the large duct type and the small duct type. Currently available targeted treatments are mainly for the small duct type iCCA as this type harbours actionable mutations, such as IDH mutations and FGFR2 fusions. Therefore, an accurate diagnosis is essential for the clinical management of iCCA; and for this reason, a multidisciplinary approach is highly recommended due to the tumour heterogeneity of iCCA. Finally, a genomic analysis based on iCCA subtypes would also be hugely beneficial, as the results may be easily translated to daily practise.
Figure 1.
Epithelial membrane antigen (EMA) staining reflecting heterogeneity of the cholangiocytes. The large bile duct is lined by mucinproducing cylindrical cholangiocytes (A), which show cytoplasmic EMA expression (B). In contrast, the small duct is lined by mucin-negative cuboidal cholangiocytes (C) which demonstrate apical EMA positivity (D). The large duct intrahepatic cholangiocarcinoma (iCCA) shows EMA cytoplasmic expression (E), and the small duct type iCCA presents apical EMA positivity (F).
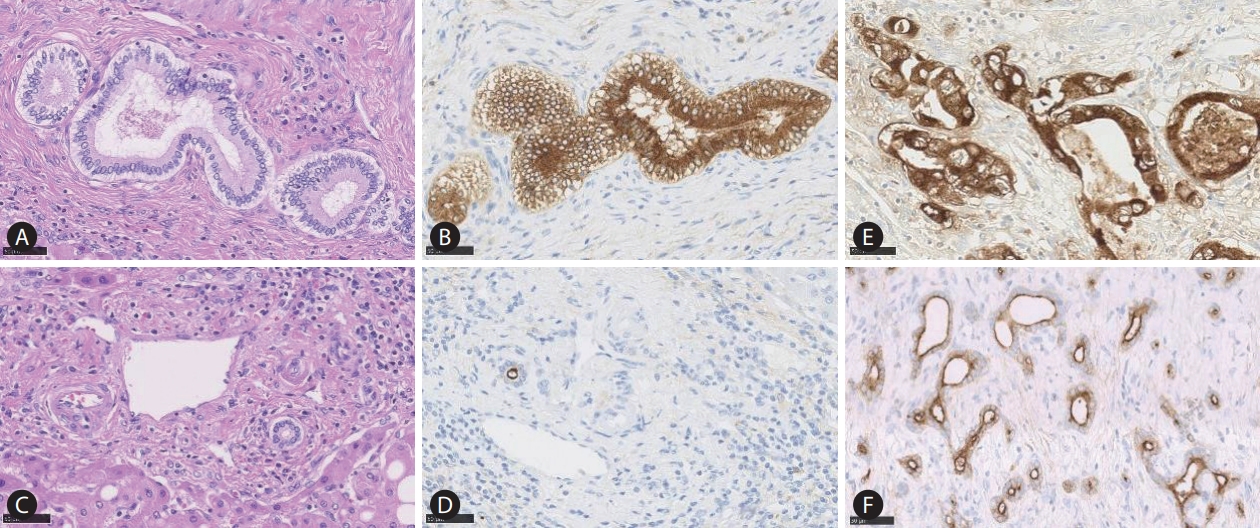
Figure 2.
Comparative macroscopic and pathological features of large and small duct type intrahepatic cholangiocarcinoma (iCCA). Macroscopically, the large duct type iCCA shows periductal infiltrating (PI) type (A), or mixed PI and mass-forming pattern (B). The tumour shows a clear glandular structure with solid fibrosis (C; HE staining, ×40). Perineural invasion (D) and lymphatic invasion (E) are also shown (HE staining, ×40). The small duct type iCCA presents with a mass-forming growth pattern (F; HE staining, ×40). Pathologically, the tumour shows variation of ductular configurations (G, H; HE staining, ×40).
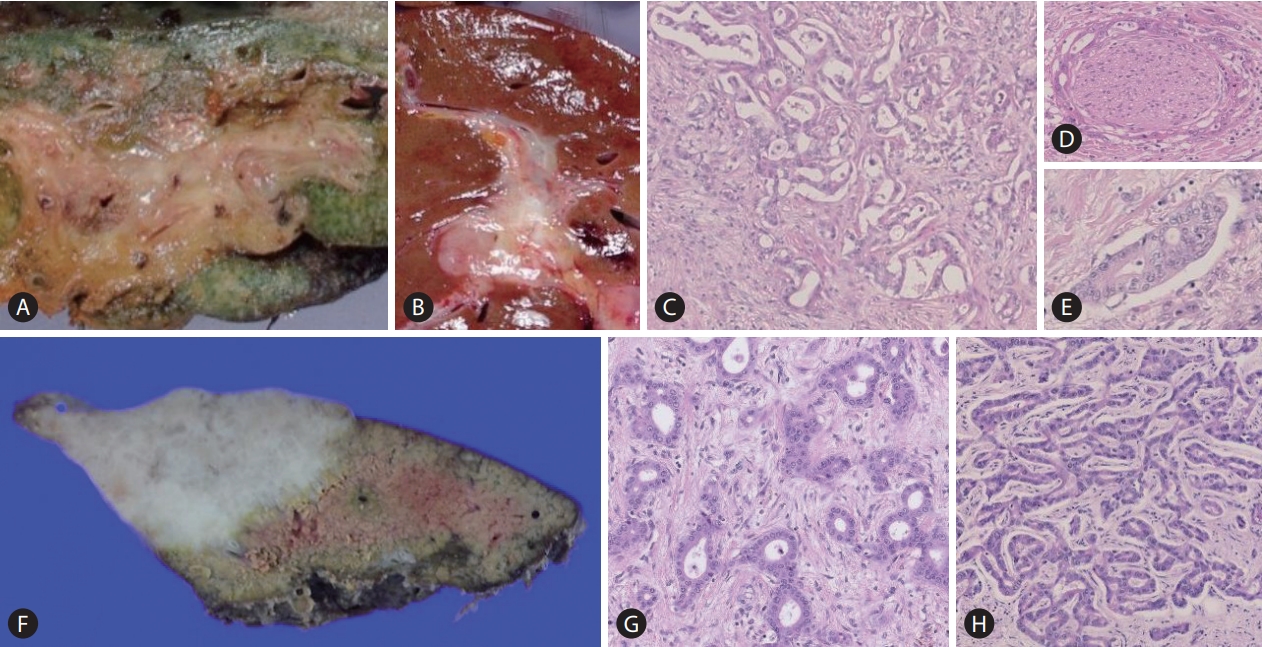
Figure 3.
Cholangiolocellular carcinoma (CLC) diagnosis and categorization. Diagnosis: CLC is a tumour comprising more than 80% ductular configuration. Epithelial membrane antigen (EMA) shows an apical expression in the ductular area. Categorization: CLC with hepatocytic differentiation is defined as combined hepatocellular carcinoma (HCC)-cholangiocarcinoma (CCA), and the remaining CLC is categorized into the small duct type intrahepatic cholangiocarcinoma (iCCA).

Figure 4.
The pathological features of large duct intrahepatic cholangiocarcinoma (iCCA) (A; Hematoxylin and Eosin [H&E] staining, ×40) are very similar to those of pancreatic cancer (B; H&E staining, ×40). The small duct type iCCA (C; H&E staining, ×10) mimics breast cancer liver metastasis (D; H&E staining, ×10).

Table 1.
Clinicopathological comparison between small and large duct type iCCA
Table 2.
Interpretation of reported molecular data based on iCCA subtypes
| Study | Category | Large duct type | Small duct type |
|---|---|---|---|
| Ahn et al. [20] (2019) | NA sequencing and pathology | Subclass B (bile-duct type pathology, worse prognosis, KRAS) | Subclass A (cholangiolar type, IDH1, FGFR2) |
| Liau et al. [19] (2014) | Pathology (cholangiolar type vs. bile duct type) | Bile duct type, KRAS | Cholangiolar type, IDH1/2 |
| Akita et al. [17] (2017) | Pathology (peripheral type vs. perihilar type) | Perihilar type, SMAD4 | Peripheral type, viral hepatitis, IDH1, BAP1 |
| Bekaii-Saab et al. [3] (2021) | Location (intra-hepatic vs. extra-hepatic) | KRAS, TP53, CDKN2A | IDH1, FGFR2, BAP1 |
| Jusakul et al. [21] (2017) | Aetiology (fluke-negative or fluke-positive) | Fluke-positive (group 1), TP53 | Fluke-negative and intrahepatic CCA (group 4), IDH1/2, FGFR, BAP1 |
| Boerner et al. [22] (2021) | Prognosis | Worse prognosis, TP53, KRAS, CDKN2A | |
| Sia et al. [23] (2013) | Integrative genomic analysis | Proliferation (P1) (worse prognosis, KRAS) | |
| Andersen et al. [24] (2012) | Genomic and genetics analysis | KRAS (poor survival group) | |
| Farshidfar et al. [26] (2017) | Integrative genomic analysis | IDH mutant subgroup, histological spectrum |
Abbreviations
BAP1
BRCA associated protein 1
BSG
the British Society of Gastroenterology
CCA
cholangiocarcinoma
CDKN2A
cyclin-dependent kinase inhibitor 2A
cHCC
combined hepatocellular carcinoma
CLC
cholangiolocellular carcinoma
dCCA
distal CCA
eCCA
extra-hepatic CCA
EMA
epithelial membrane antigen
ESMO
European Society for Medical Oncology
FGFR
fibroblast growth factor receptor
HCC
hepatocellular carcinoma
iCCA
intrahepatic CCA
ICIs
immune checkpoint inhibitors
IDH
isocitrate dehydrogenase
ILCA
the International Liver Cancer Association
K19
Keratin 19
Li-RADS
Liver image in reporting and data system
MF
mass forming
NCCN
National Comprehensive Cancer Network
OS
overall survival
pCCA
perihilar CCA
PI
periductal infiltrating
WHO
World Health Organization
REFERENCES
1. Nakanuma Y, Klimstra D, Komuta M, Zen Y. Intrahepatic cholangiocarcinoma. WHO classification of tumours of the digestive system. 5 ed. Lyon: IARC; 2019. p. 254-259.
2. Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol 2020;17:557-588.




3. Bekaii-Saab TS, Bridgewater J, Normanno N. Practical considerations in screening for genetic alterations in cholangiocarcinoma. Ann Oncol 2021;32:1111-1126.


4. Tsujie M, Iwai T, Kubo S, Ura T, Hatano E, Sakai D, et al. Fibroblast growth factor receptor 2 (FGFR2) fusions in Japanese patients with intrahepatic cholangiocarcinoma. Jpn J Clin Oncol 2021;51:911-917.




5. Javle M, Bekaii-Saab T, Jain A, Wang Y, Kelley RK, Wang K, et al. Biliary cancer: utility of next-generation sequencing for clinical management. Cancer 2016;122:3838-3847.



6. Lowery MA, Ptashkin R, Jordan E, Berger MF, Zehir A, Capanu M, et al. Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: potential targets for intervention. Clin Cancer Res 2018;24:4154-4161.




7. Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, AlRajabi R, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, openlabel, phase 2 study. Lancet Oncol 2020;21:671-684.



8. Zhu AX, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, et al. Final overall survival efficacy results of ivosidenib for patients with advanced cholangiocarcinoma with IDH1 mutation: the phase 3 randomized clinical ClarIDHy trial. JAMA Oncol 2021;7:1669-1677.



9. Javle M, Roychowdhury S, Kelley RK, Sadeghi S, Macarulla T, Weiss KH, et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol Hepatol 2021;6:803-815.


10. Bagante F, Spolverato G, Cucchetti A, Gani F, Popescu I, Ruzzenente A, et al. Defining when to offer operative treatment for intrahepatic cholangiocarcinoma: a regret-based decision curves analysis. Surgery 2016;160:106-117.


11. Farges O, Fuks D, Boleslawski E, Le Treut YP, Castaing D, Laurent A, et al. Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 study group. Ann Surg 2011;254:824-829 discussion 830.

12. Moeckli B, Ivanics T, Claasen M, Toso C, Sapisochin G. Recent developments and ongoing trials in transplant oncology. Liver Int 2020;40:2326-2344.



13. Bertuccio P, Malvezzi M, Carioli G, Hashim D, Boffetta P, El-Serag HB, et al. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol 2019;71:104-114.


14. Komuta M. Histological heterogeneity of primary liver cancers: clinical relevance, diagnostic pitfalls and the pathologist’s role. Cancers (Basel) 2021;13:2871.



15. Komuta M, Govaere O, Vandecaveye V, Akiba J, Van Steenbergen W, Verslype C, et al. Histological diversity in cholangiocellular carcinoma reflects the different cholangiocyte phenotypes. Hepatology 2012;55:1876-1888.


16. Kudo M, Izumi N, Kokudo N, Sakamoto M, Shiina S, Takayama T, et al. Report of the 21st nationwide follow-up survey of primary liver cancer in Japan (2010-2011). Hepatol Res 2021;51:355-405.



17. Akita M, Fujikura K, Ajiki T, Fukumoto T, Otani K, Azuma T, et al. Dichotomy in intrahepatic cholangiocarcinomas based on histologic similarities to hilar cholangiocarcinomas. Mod Pathol 2017;30:986-997.



18. Hayashi A, Misumi K, Shibahara J, Arita J, Sakamoto Y, Hasegawa K, et al. Distinct clinicopathologic and genetic features of 2 histologic subtypes of intrahepatic cholangiocarcinoma. Am J Surg Pathol 2016;40:1021-1030.


19. Liau JY, Tsai JH, Yuan RH, Chang CN, Lee HJ, Jeng YM. Morphological subclassification of intrahepatic cholangiocarcinoma: etiological, clinicopathological, and molecular features. Mod Pathol 2014;27:1163-1173.



20. Ahn KS, O’Brien D, Kang YN, Mounajjed T, Kim YH, Kim TS, et al. Prognostic subclass of intrahepatic cholangiocarcinoma by integrative molecular-clinical analysis and potential targeted approach. Hepatol Int 2019;13:490-500.



21. Jusakul A, Cutcutache I, Yong CH, Lim JQ, Huang MN, Padmanabhan N, et al. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov 2017;7:1116-1135.




22. Boerner T, Drill E, Pak LM, Nguyen B, Sigel CS, Doussot A, et al. Genetic determinants of outcome in intrahepatic cholangiocarcinoma. Hepatology 2021;74:1429-1444.




23. Sia D, Hoshida Y, Villanueva A, Roayaie S, Ferrer J, Tabak B, et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology 2013;144:829-840.



24. Andersen JB, Spee B, Blechacz BR, Avital I, Komuta M, Barbour A, et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology 2012;142:1021-1031.e15.



25. Israel MA, Danziger N, McGregor KA, Murugesan K, Gjoerup O, Sokol ES, et al. Comparative genomic analysis of intrahepatic cholangiocarcinoma: biopsy type, ancestry, and testing patterns. Oncologist 2021;26:787-796.




26. Farshidfar F, Zheng S, Gingras MC, Newton Y, Shih J, Robertson AG, et al. Integrative genomic analysis of cholangiocarcinoma identifies distinct IDH-mutant molecular profiles. Cell Rep 2017;18:2780-2794.


28. Komuta M, Spee B, Vander Borght S, De Vos R, Verslype C, Aerts R, et al. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology 2008;47:1544-1556.


29. Sempoux C, Kakar S, Kondo F, Schirmacher P. Combined hepatocellular- cholangiocarcinoma and undifferentiated primary liver carcinoma. WHO classification of tumours of the digestive system. 5 ed. Lyon: IARC; 2019. p. 260-262.
30. Moeini A, Sia D, Zhang Z, Camprecios G, Stueck A, Dong H, et al. Mixed hepatocellular cholangiocarcinoma tumors: cholangiolocellular carcinoma is a distinct molecular entity. J Hepatol 2017;66:952-961.


31. Komuta M, Yeh MM. A Review on the update of combined hepatocellular cholangiocarcinoma. Semin Liver Dis 2020;40:124-130.


32. Akiba J, Nakashima O, Hattori S, Tanikawa K, Takenaka M, Nakayama M, et al. Clinicopathologic analysis of combined hepatocellular-cholangiocarcinoma according to the latest WHO classification. Am J Surg Pathol 2013;37:496-505.


33. Park SH, Lee SS, Yu E, Kang HJ, Park Y, Kim SY, et al. Combined hepatocellular-cholangiocarcinoma: gadoxetic acid-enhanced MRI findings correlated with pathologic features and prognosis. J Magn Reson Imaging 2017;46:267-280.



34. Elsayes KM, Kielar AZ, Chernyak V, Morshid A, Furlan A, Masch WR, et al. LI-RADS: a conceptual and historical review from its beginning to its recent integration into AASLD clinical practice guidance. J Hepatocell Carcinoma 2019;6:49-69.


35. Choi SH, Jeon SK, Lee SS, Lee JM, Hur BY, Kang HJ, et al. Radiopathologic correlation of biphenotypic primary liver cancer (combined hepatocellular cholangiocarcinoma): changes in the 2019 WHO classification and impact on LI-RADS classification at liver MRI. Eur Radiol 2021;31:9479-9488.



36. Gigante E, Ronot M, Bertin C, Ciolina M, Bouattour M, Dondero F, et al. Combining imaging and tumour biopsy improves the diagnosis of combined hepatocellular-cholangiocarcinoma. Liver Int 2019;39:2386-2396.



37. Durnez A, Verslype C, Nevens F, Fevery J, Aerts R, Pirenne J, et al. The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma. A possible progenitor cell origin. Histopathology 2006;49:138-151.


38. Govaere O, Komuta M, Berkers J, Spee B, Janssen C, de Luca F, et al. Keratin 19: a key role player in the invasion of human hepatocellular carcinomas. Gut 2014;63:674-685.



39. Fong ZV, Brownlee SA, Qadan M, Tanabe KK. The clinical management of cholangiocarcinoma in the United States and Europe: a comprehensive and evidence-based comparison of guidelines. Ann Surg Oncol 2021;28:2660-2674.



40. Bedossa P, Poitou C, Veyrie N, Bouillot JL, Basdevant A, Paradis V, et al. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology 2012;56:1751-1759.


41. De Martin E, Rayar M, Golse N, Dupeux M, Gelli M, Gnemmi V, et al. Analysis of liver resection versus liver transplantation on outcome of small intrahepatic cholangiocarcinoma and combined hepatocellular-cholangiocarcinoma in the setting of cirrhosis. Liver Transpl 2020;26:785-798.



42. Sapisochin G, Facciuto M, Rubbia-Brandt L, Marti J, Mehta N, Yao FY, et al. Liver transplantation for “very early” intrahepatic cholangiocarcinoma: International retrospective study supporting a prospective assessment. Hepatology 2016;64:1178-1188.



43. Lamarca A, Ross P, Wasan HS, Hubner RA, McNamara MG, Lopes A, et al. Advanced intrahepatic cholangiocarcinoma: post hoc analysis of the ABC-01, -02, and -03 clinical trials. J Natl Cancer Inst 2020;112:200-210.



44. Lee SH, Simoneau EB, Karpinets T, Futreal PA, Zhang J, Javle M, et al. Genomic profiling of multifocal intrahepatic cholangiocarcinoma reveals intraindividual concordance of genetic alterations. Carcinogenesis 2021;42:436-441.




45. Lamarca A, Santos-Laso A, Utpatel K, La Casta A, Stock S, Forner A, et al. Liver metastases of intrahepatic cholangiocarcinoma: implications for an updated staging system. Hepatology 2021;73:2311-2325.




46. Gutiérrez-Larrañaga M, González-López E, Roa-Bautista A, Rodrigues PM, Díaz-González Á, Banales JM, et al. Immune checkpoint inhibitors: the emerging cornerstone in cholangiocarcinoma therapy? Liver Cancer 2021;10:545-560.




47. Feng K, Liu Y, Zhao Y, Yang Q, Dong L, Liu J, et al. Efficacy and biomarker analysis of nivolumab plus gemcitabine and cisplatin in patients with unresectable or metastatic biliary tract cancers: results from a phase II study. J Immunother Cancer 2020;8:e000367.



48. Ueno M, Ikeda M, Morizane C, Kobayashi S, Ohno I, Kondo S, et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: a non-randomised, multicentre, open-label, phase 1 study. Lancet Gastroenterol Hepatol 2019;4:611-621.


- TOOLS
-
METRICS

- ORCID iDs
-
Mina Komuta

https://orcid.org/0000-0002-3999-1417 - Related articles
-
Intrahepatic cholangiocarcinoma arising in Caroli's disease2014 December;20(4)
Intrahepatic cholangiocarcinoma with predominant ductal plate malformation pattern2014 June;20(2)
Intrahepatic cholangiocarcinoma associated with Clonorchis sinensis infection2009 December;15(4)
Intrahepatic Cholangiocarcinoma associated with Hepatolithiasis2007 September;13(3)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



