| Clin Mol Hepatol > Volume 29(Suppl); 2023 > Article |
|
ABSTRACT
ACKNOWLEDGMENTS
FOOTNOTES
SUPPLEMENTARY MATERIAL
Supplementary Table 1.
Supplementary Table 2.
Figure 1.
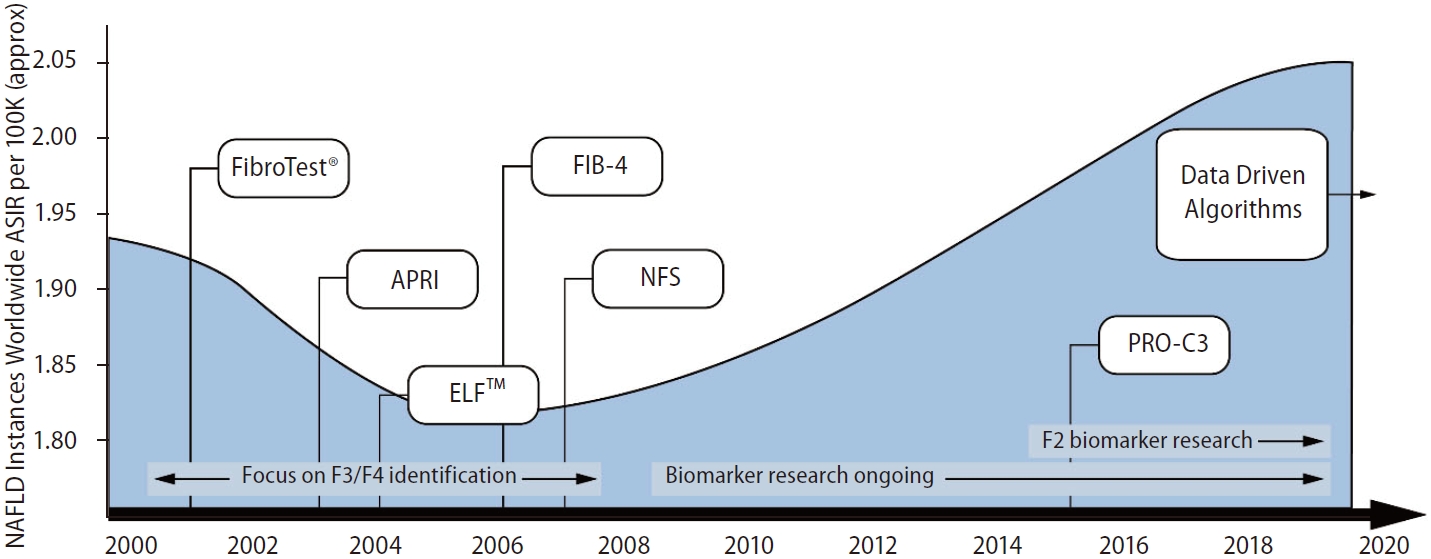
Figure 2.
Table 1.
| Performance |
Noninvasive blood biomarker |
|||||
|---|---|---|---|---|---|---|
| ELF™ [35] | FIB-4 [32] | NFS [32] | APRI [32] | FibroTest® [33] | ||
| AUC value | 0.83 | 0.80 | 0.78 | 0.75 | 0.77 | |
| Sensitivity | 0.42 | 0.32 | 0.43 | 0.33 | 0.72 | |
| Specificity | 0.95 | 0.96 | 0.88 | 0.91 | 0.69 | |
| PPV | 0.85 | 0.66 | 0.67 | 0.56 | NR | |
| NPV | 0.71 | 0.85 | 0.89 | 0.79 | NR | |
| Notable differences | ||||||
| Age included in algorithm | √ | √ | √ | √ | ||
| Score calculated from routine blood and anthropometric measurements* | √ | √ | √ | |||
| Additional costs beyond routine blood tests incurred | √ | √ | ||||
| Utility for high prevalence setting only | √ | √ | √ | √ | √ | |
NAFLD, non-alcoholic fatty liver disease; AUC, area under the curve; PPV, positive predictive value; NPV, negative predictive value; NR, not reported; ELF™, enhanced liver fibrosis; FIB-4, fibrosis-4; NFS, nonalcoholic fatty liver disease fibrosis score; APRI, aspartate transaminase to platelet ratio index. *Online calculators for FIB-4, NFS, and APRI are available: FIB-4: e.g., https://gps.northcentrallondon.icb.nhs.uk/fib-4-calculator and https://www.hepatitisc.uw.edu/page/clinical-calculators/fib-4.
APRI: e.g., https://www.hepatitisc.uw.edu/page/clinical-calculators/apri and https://www.omnicalculator.com/health/apri.
Table 2.
| Biomarkers | Cut-off values | AUC | Summary sensitivity (%) | Summary specificity (%) | Summary PPV (%) | Summary NPV (%) |
|---|---|---|---|---|---|---|
| APRI [32] | 0.43–1.50 | 0.70 | 59.3 (33.3–71.1) | 77.1 (66.2–90.6) | 67.5 (61.1–74.3) | 70.6 (57.6–87.5) |
| FIB-4 [32] | 0.37–3.25 | 0.75 | 64.4 (54.4–77.8) | 70.0 (60.0–87.5) | 73.3 (66.2–77.8) | 60.6 (40.5–74.2) |
| FibroTest® [33] | 0.30–0.75 | 0.77 | 56.0 (45.0–66.0) | 77.0 (74.0–80.0) | NR | NR |
| NFS [32],* | –1.1 | 0.72 | 66.5 (60.9–70.1) | 82.5 (68.7–96.3) | 81.7 (76.6–86.7) | 73.6 (61.1–86.0) |
| ELF™ [35] | 7.7† | 0.81 | Sensitivity=0.96 | Specificity=0.12 | PPV=0.42 | NPV=0.83 |
Values are presented as mean (range).
ELF™, enhanced liver fibrosis test; FIB-4, fibrosis-4; APRI, aspartate transaminase to platelet ratio index; NFS, nonalcoholic fatty liver disease fibrosis score; AUC, area under the curve; PPV, positive predictive value; NPV, negative predictive value; NR, not recorded.
* Two studies were used for to assess the performance of NFS for significant fibrosis. One cut point was reported.
† Manufacturers recommended cut-off value for moderate fibrosis. [50]
Table 3.
| Study | Study design, duration & numbers recruited | Relevant drug for NASH | Patient group | Fibrosis marker | Baseline | Follow-up | Change in mean | Change in serum biomarker score |
|---|---|---|---|---|---|---|---|---|
| Newsome et al. [63] (2021) | Phase 2, double-blind, randomised, placebo-controlled; 72 weeks; n=320 | Semaglutide | 0.4 mg | Mean fibrosis stagea,* (SD) | 2.2 (0.6) | 1.7 (0.4) | –0.5 | |
| Mean ELF™ scored,f | 9.9±1.0 | 9.2b | –0.56c | |||||
| Mean VCTE reading, kPae | 11.5±87.1 | 7.68g | –3.82 | |||||
| Placebo | Mean fibrosis stagea,* (SD) | 2.2 (0.6) | 2.0 (0.4) | –0.2 | ||||
| Mean ELF™ scored,f | 9.6±0.9 | 9.77b | 0.01c | |||||
| Mean VCTE reading, kPae | 8.7±90.0 | 10.84g | 2.14b | |||||
| Friedman et al. [64] (2018) | Phase 2b, double- blind, randomised, placebo-controlled; 52 weeks; n=288 | Cenicriviroc | 150 mg | Mean fibrosis stage* (SD) | 2.1 (0.5) | 1.9 (0.4) | –0.2 | |
| Median NFS score (min, max) | –0.942 (–4.55, 1.27) | –0.942 (–4.55, 1.27) | –0.942 (–4.55, 1.27) | |||||
| Median FIB-4 score (min, max) | 1.239 (0.38, 4.20) | 1.375 (0.42, 5.26) | 0.080 (–1.81, 2.38) | |||||
| Median APRI score, (min, max) | 0.470 (0.20, 3.12) | 0.539 (0.15, 3.45) | 0.024 (–1.30, 1.49) | |||||
| Median ELF™§ (min, max) | –0.892 (–2.70, 1.27) | –0.828 (–2.50, 1.08) | 0.023 (–1.98, 1.65) | |||||
| Placebo | Mean fibrosis score* (SD) | 2.0 (0.5) | 2.1 (0.4) | 0.1 | ||||
| Median NFS score (min, max) | –1.223 (–4.81, 2.46) | –1.190 (–4.27, 2.34) | 0.102 (–1.74, 1.37) | |||||
| Median FIB-4 score (min, max) | 1.303 (0.40, 4.14) | 1.242 (0.36, 5.32) | 0.006 (–1.18, 3.11) | |||||
| Median APRI score, (min, max) | 0.568 (0.15, 2.26) | 0.538 (0.13, 3.71) | –0.031 (–0.82, 3.46) | |||||
| Median ELF™§(min, max) | –0.893 (–2.20, 1.62) | –1.003 (–2.53, 2.07) | –0.113 (–1.21, 1.60) | |||||
| Francque et al. [65] (2021) | Phase 2b, double-blind, randomised, placebo-controlled; 24 weeks; n=247 | Lanifibranor | 1,200 mg | Mean fibrosis score (SD)d,h,* | 2.1±0.8 | NR | NR | |
| Median ELF™ scorei (IQR) | NR | NR | 0.11 (–0.04 to 0.26) | |||||
| Median FIB-4 (IQR) | NR | NR | 0.03 (–0.13 to 0.19) | |||||
| Median PRO-C3, μg/L (IQR) | NR | NR | –1.79 (–3.07 to –0.52) | |||||
| Mean VCTE reading, kPa (SD) | 9.99 (5.46) | NR | –1.01 (3.88) | |||||
| Placebo | Mean fibrosis score (SD)d,h,* | 2.0±0.8 | NR | NR | ||||
| Median ELF™ scorei (IQR) | NR | NR | –0.08 (–0.23 to 0.06) | |||||
| Median FIB-4 (IQR) | NR | NR | 0.03 (–0.19 to 0.13) | |||||
| Median PRO-C3, μg/L (IQR) | NR | NR | –1.01 (–2.30 to 0.28) | |||||
| Mean VCTE reading, kPa (SD) | 9.96 (4.89) | NR | –0.66 (3.04) | |||||
| Harrison et al. [66] (2020) | Phase 2b, double-blind, randomised, placebo-controlled; 52 weeks; n=392 | MSDC-0602K | 250 mg | Mean fibrosis stagea,* (SD) | 2.10 (0.53) | NR | –0.1 | Reported as: the average effect of the combined highest doses relative to placebo on ELF™ FIB-4, FibroTest®, and CK-18 was a reduction of 0.21 (95% CI −0.39 to −0.03) SDs at 6 months and 0.17 (95% CI −0.37 to 0.02) SDs at 12 months. |
| Mean APRI score (SD) | 0.604 (0.4385) | NR | ||||||
| Mean ELF™ score (SD) | 9.80 (1.052) | NR | ||||||
| Mean FIB-4 score (SD) | 1.58 (0.909) | NR | ||||||
| Mean FibroTest® (SD) | 0.33 (0.192) | NR | ||||||
| Placebo | Mean fibrosis stagea,* (SD) | 2.2 (0.6) | NR | 0.1 | ||||
| Mean APRI score (SD) | 0.540 (0.2896) | NR | ||||||
| Mean ELF™ score (SD) | 9.6 (0.850) | NR | ||||||
| Mean FIB-4 score (SD) | 1.38 (0.688) | NR | ||||||
| Mean FibroTest® (SD) | 0.31 (0.197) | NR | ||||||
| Armstrong et al. [67] (2016) | Phase 2, double-blind, randomised, placebo-controlled; 48 weeks; n=52 | Liraglutide | 1.8 mg | Mean fibrosis stage† (SD) | 2.3 (0.9) | NR | –0.2 (0.8) | |
| Mean ELF™ score (SD) | 9.3 (SD) | NR | –0.3 (0.8) | |||||
| Placebo | Mean fibrosis stage† (SD) | 2.3 (1.3) | NR | 0.2 (1.0) | ||||
| Mean ELF™ score (SD) | 9.4 (1.3) | NR | 0.1 (0.8) | |||||
| Chalasani et al. [72] (2020) | Phase 2b, double-blind, randomised, placebo-controlled; 52 weeks; n=162 | Belapectin | 8 mg/kg | Mean fibrosis stagea,‡ (SD) | 4.0b | 3.75a (1.3) | –0.25b | |
| Mean ELF™ score (SD) | 10.64 (1.16) | NR | 0.50 (0.78) | |||||
| Mean FibroTest® score (SD) | NR | NR | 0.01 (0.02) | |||||
| Mean VCTE reading, kPa (SD) | 29.3 (14.9) | NR | –2.34 (10.8) | |||||
| Placebo | Mean fibrosis stagea,‡ (SD) | 4.0b | 3.7a (1.3) | –0.3b | ||||
| Mean ELF™ score (SD) | 10.81 (1.1) | NR | 0.37 (0.63) | |||||
| Mean FibroTest® score (SD) | NR | NR | 0.03 (0.02) | |||||
| Mean VCTE reading, kPa (SD) | 29.9 (17.8) | NR | –0.47 (18.6) | |||||
| Harrison et al. [68] (2021) | Phase 2, double blind, randomised, placebo-controlled; 24 weeks; n=78 | Aldafermin | 1 mg | Mean fibrosis stagea,* (SD) | 2.5a (0.7) | NR | NRj | |
| Mean ELF™ score (SD) | 9.8 (0.8) | NR | –0.2 (0.5) | |||||
| Mean PRO-C3 score, μg/L (SD) | 17.5 (8.4) | NR | –5.4 (6.2) | |||||
| Placebo | Mean fibrosis stagea,* (SD) | 2.4 (0.7) | NR | NRj | ||||
| Mean ELF™ score (SD) | 9.9 (1.0) | NR | 0 (0.6) | |||||
| Mean PRO-C3 score, μg/L (SD) | 17.1 (7.0) | NR | –1.2 (6.2) | |||||
| Harrison et al. [69] (2021) | Phase 2a, double blind, randomised, placebo-controlled; 12 weeks; n=80 | Efruxifermin | 70 mg | Mean fibrosis stagea,* (SD) | 2.0 (0.4) | NR | NR | |
| Mean ELF™ score (SD) | 9.5 (0.8) | NR | 9.3b,k | |||||
| Mean PRO-C3 score, μg/L (SD) | 17.2 (5.9) | NR | 10.0b,k | |||||
| Placebo | Mean fibrosis stagea,* (SD) | 2.0 (0.5) | NR | NR | ||||
| Mean ELF™ score (SD) | 9.5 (1.0) | NR | 9.5b,k | |||||
| Mean PRO-C3 score, μg/L (SD) | 16.1 (6.7) | NR | 15.0b,k | |||||
| Loomba et al. [73] (2021) | Phase 2b, double blind, randomised, placebo-controlled; 48 weeks; n=392 | Cilofexor Firsocostat | Cilofexor 30 mg | Biopsy confirmed F3/F4* | n=76 (98%) | NR | NR | |
| Firsocostat 20 mg | Median ELF™ score (IQR) | 10.0 (9.4, 10.7) | NR | –0.0 (–0.2, 0.20) | ||||
| Median VCTE reading, kPa (IQR) | 15.7 (10.9, 22.2) | NR | –4.2 (–6.5, –1.9) | |||||
| Harrison et al. [70] (2019) | Phase 2, double blind, randomised, placebo-controlled; 36 weeks; n=125 | Resmetriom | 80 mg | Mean fibrosis stagea,* (SD) | 1.6 (0.3) | NR | NR | |
| Mean ELF™ score (SD) | 9.2 (0.9) | NR | –0.38m (0.09) | |||||
| Mean PRO-C3 score, μg/L (SD) | 17.8 (10.3) | NR | –2.2p (2.1); –6.5q (3.5) | |||||
| Placebo | Mean fibrosis stagea,* (SD) | 1.6 (0.3) | NR | NR | ||||
| Mean ELF™ score (SD) | 9.2 (1.0) | NR | 0.02l (0.12) | |||||
| Mean PRO-C3 score, μg/L (SD) | 16.2 (59.0) | NR | 7.4n (3.1); 14.9o (5.6) | |||||
| Ratziu et al. [71] (2016) | Phase 2, double blind, randomised, placebo-controlled; 52 weeks; n=276 | Elafibranor | 120 mg | Mean fibrosis stage* (SD) | 1.7 (0.9) | NR | NR | |
| Mean NFS score (SD) | NR | NR | –0.25b | |||||
| Mean FibroTest® (SD) | NR | NR | –0.07b | |||||
| Placebo | Mean fibrosis stage* (SD) | 1.5 (1.0) | NR | NR | ||||
| Mean NFS score (SD) | NR | NR | –0.01b | |||||
| Mean FibroTest® (SD) | NR | NR | –0.01b | |||||
| Harrison et al. [74] (2020) | Phase III (STELLAR-4), double blind, randomised, placebo-controlled; 48 weeks; n=877 | Selonsertib | 18 mg | Mean fibrosis stagea,* (SD) | 4.0 (1.8) | 3.7 (1.4) | –0.3b | |
| Median ELF™ score (IQR) | 10.61 (10.04 to 11.34) | 10.73 (10.07 to 10.51) | 0.10b | |||||
| Median FibroTest® (IQR) | 0.58 (0.44 to 0.73) | 0.58 (0.40 to 0.75) | NC | |||||
| Median APRI score (IQR) | 0.8 (0.6 to 1.2) | 0.8 (0.5 to 1.3) | NC | |||||
| Median FIB-4 score (IQR) | 2.55 (1.76 to 3.62) | 2.65 (1.74 to 3.76) | 0.10b | |||||
| Median NFS score (IQR) | 0.659 (–0.119 to 1.472) | 0.816 (0.031 to 1.574) | 0.157b | |||||
| Median VCTE reading, kPa (IQR) | 21.10 (14.7 to 28.8) | 19.4 (14.3 to 27.3) | –1.7b | |||||
| Placebo | Mean fibrosis stagea,* (SD) | 3.7 (1.4) | 3.8 (1.5) | 0.10b | ||||
| Median ELF™ score (IQR) | 10.67(10.05 to 11.16) | 10.66 (10.14 to 11.26) | –0.01b | |||||
| Median FibroTest® (IQR) | 0.59 (0.40 to 0.77) | 0.57 (0.39 to 0.73) | –0.02b | |||||
| Median APRI score (IQR) | 0.8 (0.6 to 1.2) | 0.7 (0.5 to 1.2) | –0.1b | |||||
| Median FIB-4 score (IQR) | 2.50 (1.81 to 3.66) | 2.50 (1.65 to 3.67) | NC | |||||
| Median NFS score (IQR) | 0.682 (–0.304 to 1.450) | 0.774 (–0.241 to 1.595) | 0.092b | |||||
| Median VCTE reading, kPa (IQR) | 20.00 (14.4 to 26.7) | 19.30 (13.8 to 26.7) | 0.70b | |||||
| Loomba et al. [75] (2018) | Phase 2, double blind, randomised, de facto placebo-controlled; 24 weeks; n=72 | Selonsertib±Simtuzumab | Selonsertib 18 mg±Simtuzumab | Biopsy confirmed F3* | n=21 (66%) | Improvement n=13 (43%); Cirrhosis n=1 (3%) | ||
| Median ELF™ score (IQR) | NR | NR | 0.02 (–0.34 to 0.52) | |||||
| Median FibroTest® (IQR) | NR | NR | –0.01 (–0.03 to 0.03) | |||||
| Median VCTE reading, kPa (IQR) | NR | NR | 0.2 (–3.50 to 1.40) | |||||
| Simtuzumab | Biopsy confirmed F3* | n=6 (60%) | Improvement n=2 (20%); Cirrhosis n=2 (20%) | |||||
| Median ELF™ score (IQR) | NR | NR | –0.13 (–0.35 to 0.05) | |||||
| Median FibroTest® (IQR) | NR | NR | 0.01 (–0.04 to 0.05) | |||||
| Median VCTE reading, kPa (IQR) | NR | NR | –0.50 (–3.80 to 3.4) |
NR, not reported; NC, no change; ELF™, enhanced liver fibrosis; FIB-4, fibrosis-4; NFS, NAFLD fibrosis score; APRI, aspartate transaminase to platelet ratio index; PRO-C3, Type III collagen marker of the N-terminal pro-peptide; SD, standard deviation; IQR, interquartile range; VCTE, vibration controlled transient elastography.
a Mean not provided, calculation made using data provided in the manuscript tables and supplementary information.
c Change in biomarker score is the change reported in the research paper and not the exact difference between baseline and follow-up.56
f An ELF™ score greater than 9.8 indicates a moderate risk of advanced fibrosis, and a score of greater than 11.3 denotes a high risk of advanced fibrosis.
i An ELF™ score of less than 7.7 indicates none to mild fibrosis, and a score of 11.3 or greater indicates cirrhosis.
j Improvement/no improvement or worsening reported, unable to calculate changes in fibrosis stage as data is not provided.
Abbreviations
REFERENCES
- TOOLS
-
METRICS

- ORCID iDs
-
Tina Reinson

https://orcid.org/0000-0002-2436-1906 - Related articles
-
Non-invasive biomarkers of liver fibrosis in non-alcoholic fatty liver disease2023 April;29(2)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement1
Supplement1 Print
Print



