Interaction between sarcopenia and nonalcoholic fatty liver disease
Article information
Abstract
Sarcopenia and nonalcoholic fatty liver disease (NAFLD) are common health problems related to aging. Despite the differences in their diagnostic methods, several cross-sectional and longitudinal studies have revealed the close link between sarcopenia and NAFLD. Sarcopenia and NAFLD are linked by several shared pathogenetic mechanisms, including insulin resistance, hormonal imbalance, systemic inflammation, myostatin and adiponectin dysregulation, nutritional deficiencies, and physical inactivity, thus implicating a bidirectional relationship between sarcopenia and NAFLD. However, there is not sufficient data to support a direct causal relationship between sarcopenia and NAFLD. Moreover, it is currently difficult to conclude whether sarcopenia is a risk factor for nonalcoholic steatohepatitis (NASH) or is a consequence of NASH. Therefore, this review intends to touch on the shared common mechanisms and the bidirectional relationship between sarcopenia and NAFLD.
INTRODUCTION
The global epidemic of obesity and metabolic syndrome in an aging population has led to growing health problems including nonalcoholic fatty liver disease (NAFLD) and sarcopenia. Sarcopenia is defined as the progressive and generalized loss of skeletal muscle mass, strength, and/or function with a risk of adverse outcomes such as physical disability, hospitalization, and mortality [1,2]. Despite the differences in their diagnostic methods, several studies have revealed the close link between sarcopenia and NAFLD [3-16]. This review focuses on the shared mechanisms and a bidirectional relationship between sarcopenia and NAFLD.
OPERATIONAL DEFINITION OF SARCOPENIA
Sarcopenia, previously considered an aging-related syndrome, is now recognized as a progressive disease associated with type 2 diabetes mellitus (T2DM), metabolic syndrome, liver disease, and cardiovascular disease [17-20]. It is primarily associated with aging and secondarily with diseases mediated by systemic inflammation and insulin resistance (IR) [21]. In 2018, the European Working Group on Sarcopenia in Older People defined sarcopenia by low levels across three parameters: muscle strength, muscle quantity/quality, and physical performance. The presence of low muscle strength is the primary parameter to suspect sarcopenia, while the presence of low muscle mass (quantity) and quality are confirmatory. The coexistence of these factors represents severe sarcopenia [2]. Therefore, all these parameters enable improved understanding and awareness of sarcopenia.
SHARED MECHANISMS OF SARCOPENIA AND NAFLD
Sarcopenia and NAFLD share common underlying mechanisms, including IR, hormonal imbalance, systemic inflammation, myostatin and adiponectin dysregulation, nutritional deficiencies, and physical inactivity (Fig. 1) [22].
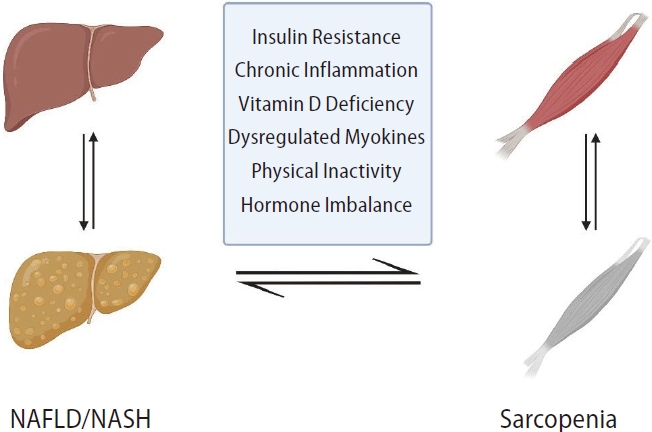
Bidirectional relationship between sarcopenia and NAFLD. NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis.
Insulin resistance
IR is the main pathologic mechanism causing both sarcopenia and NAFLD. IR results from the loss of skeletal muscle mass. It causes increased lipolysis with the consequent release of free fatty acids (FFA) from adipose tissue. IR also inhibits growth hormone (GH)/insulin growth factor-1 (IGF-1) axis that normally plays a protective role in muscle regeneration and age-related muscle loss [17,23,24]. It causes compensatory hyperinsulinemia, which leads to promotion of gluconeogenesis, upregulation of sterol regulatory element binding protein 1c, inhibition of β-oxidation, increased FFA delivery, and altered triglyceride (TG) transport. These events leads to accumulation of TGs in skeletal muscle and the liver, often referred to as ectopic fat [25,26].
Impaired suppression of gluconeogenesis promotes proteolysis and reduces protein synthesis [7], which results in age-related muscle depletion and sarcopenia [27-29]. Insulin activates the mammalian target of rapamycin (mTOR), 4E-binding protein 1, and ribosomal S6 kinase 1. These are involved in protein synthesis, maintenance of muscle mass, and skeletal muscle anabolism [30]. Skeletal muscle IR leads to increased muscle degradation with decreased mitochondrial content, function, and oxidative capacity [31]. A study demonstrated that T2DM was independently associated with sarcopenia, leading to metabolic disorders and physical disability in older adults with T2DM [32]. Furthermore, sarcopenia aggravates IR, since skeletal muscle is a primary insulin-responsive organ [33]. Likewise, myosteatosis, defined as fatty infiltration of muscle, is associated with reduced muscle function, IR, and a high risk of mortality in cirrhotic patients [34,35]. Both sarcopenia and obesity simultaneously induce more severe IR and glycemic dysregulation [33].
Chronic inflammation
Inflammation and oxidative stress have been linked to the pathogenesis of NAFLD. Intramuscular lipid accumulation induces the secretion of proinflammatory cytokines from adipose tissue and generates oxygen-free radicals in the liver by inhibiting mitochondrial function for β-oxidation, leading to lipid peroxidation. Cytokines, such as interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and transforming growth factor-β (TGF-β) induce chronic low-grade inflammation [36,37]. Compared to healthy subjects, patients with isolated steatosis and steatohepatitis had increased TNF-α levels [38]. TNF-α causes lipid accumulation in the liver through activation of de novo lipogenesis (DNL) [39]. It also stimulates nuclear factor κB, the main transcriptional factor for proinflammatory cytokines that contribute to the development of NAFLD and muscle catabolism [36,39,40]. Catabolic inflammation further worsens sarcopenia among older patients because of the release of numerous inflammatory mediators from immune cells and adipocytes that contribute to the development of IR [41]. Patients with sarcopenia demonstrate chronic inflammation, increased levels of C-reactive protein (CRP) and proinflammatory cytokines, and decreased levels of anti-inflammatory cytokines [3]. IL-6 and CRP levels are also positively associated with total body fat mass and inversely associated with appendicular lean body mass [4,42].
Vitamin D
Vitamin D is involved in the modulation of IR, NAFLD, metabolic syndrome, and sarcopenia [43]. It plays an essential role in myogenesis, myoblast proliferation and differentiation, production and growth of skeletal muscle cells, and skeletal muscle inflammation [44-47]. It exerts its effects through the nuclear vitamin D receptor (VDR), which is expressed in the liver and skeletal muscle [48,49]. Downregulation of VDR expression by vitamin D deficiency and aging may lead to sarcopenia [36]. Studies shows that subjects with sarcopenia have significantly lower vitamin D levels [6,50]. Decreased levels of vitamin D are associated with decreased muscle strength, poor muscle function, and an increased risk of sarcopenia among older adults [51]. However, vitamin D supplementation increases VDR expression in skeletal muscle, preventing the development of sarcopenia [52].
The relationship between vitamin D and NAFLD has been already acknowledged. A meta-analysis including 17 cross-sectional and case-control studies showed that patients with NAFLD had decreased levels of serum vitamin D [43]. Hypovitaminosis D was strongly associated with the presence of NAFLD independent of metabolic syndrome, T2DM, and IR [50].
Furthermore, vitamin D downregulates the expression of SREBP-1c, acetyl-coenzyme A carboxylase, and fatty acid synthase that modulate DNL, while peroxisome proliferatoractivated receptor α and carnitine palmitoyltransferase-1 that mediate hepatic fatty acid oxidation are upregulated by vitamin D [53]. An animal study demonstrated that vitamin D deficiency worsened NAFLD by activating the inflammationmediated pathway [43]. Vitamin D deficiency also causes IR via upregulation of hepatic IR, inflammatory, and oxidative stress genes [54,55]. Moreover, VDR-knockout mice spontaneously developed hepatic steatosis [55]. Most studies, to date, have shown that vitamin D plays a pivotal role in the development of sarcopenia and NAFLD. On the contrary, other studies demonstrated no significant relationship between vitamin D level and NAFLD/sarcopenia [56,57].
Myokines
Skeletal muscle is an endocrine organ that releases myokines [58,59] after muscle contraction or strength training [60]. Myokines are involved in the autocrine regulation of muscle metabolism and the paracrine/endocrine regulation of other tissues and organs including the liver, adipose tissue, and brain [61-63].
Myostatin, a member of the TGF-β family, is predominantly expressed in skeletal muscles [64,65]. It is an inhibitor of muscle mass and a key regulator of adipogenesis [65-68]. It mediates Smad 2/3 activation, inhibiting myogenesis and protein synthesis by suppressing the Akt-mediated mTOR signaling pathway [69]. This causes muscle atrophy. Muscle proteolysis is stimulated through FoxO-dependent activation of the ubiquitin-proteasome pathway and autophagy [69]. Myostatin also increases adipose tissue mass and inhibits adiponectin secretion [22,70,71]. Animal studies have demonstrated that blockage of myostatin significantly increases muscle mass, improves insulin sensitivity, and protects against liver steatosis [72,73]. Animal models have demonstrated increased expression of activin type IIB, a myostatin receptor expressed in stellate cells, in liver fibrosis [74,75]. Stellate cell cultures exposed to myostatin increase the expression of profibrotic proteins [76]. Therefore, myostatin, IR, and liver fibrogenesis are interconnected.
Irisin, an exercise-induced myokine, is inversely associated with the degree of fatty liver in obese patients and is a potential cause of sarcopenia and NAFLD. It increases energy expenditure through peroxisome proliferator- activated receptor α-dependent downstream signaling and improves insulin sensitivity and hepatic steatosis by upregulating fibroblast growth factor-21; these effects were independent of reduction in body weight and adiposity in a mouse model [77,78]. It increases glucose uptake by enhancing glucose transporter type 4 translocation and β-oxidation of FFA through AMP-activated protein kinase activation in muscle cells [79]. Irisin expression in muscle and serum irisin level are reduced in obese subjects [80].
IL-6 has a dual metabolic effect. Muscle contrations stimulate acute IL-6 release from muscles [81,82], with the levels increasing as the duration and intensity of muscle contraction increase [83,84]. IL-6 improves hepatic glucogenesis, lipolysis in adipose tissue, pancreatic β-cell viability, and insulin secretion [81,85,86]. It also enhances glucose uptake and fatty acid oxidation through adenosine monophosphate-activated protein kinase (AMPK) and phosphoinositide 3-kinase signaling processes [87,88]. However, IL-6 acts as a pro-inflmmatory cytokine in chronic inflammatory states such as obesity, infection, and cancer [89]. A study have demonstrated that increased IL-6 levels are associated with NASH, hepatic fibrosis, and IR [90].
Physical inactivity
The lack of physical activity causes loss of muscle mass and reduces energy consumption, resulting in obesity and hepatic steatosis [91]. Both sarcopenia and NAFLD are worsened by chronic inflammation, oxidative stress, and IR [92]. During exercise, production of pro-inflammatory cytokines is decreased while anti-inflammatory cytokine production, muscle protein synthesis, regeneration, and glucose uptake are increased. Physical activity mitigates the risk of sarcopenia progression [93]. Exercise can improve metabolic health status even without significant weight loss [94].
Other mechanisms
Adiponectin, a hormone secreted from adipose tissue, mediates glucose and lipid metabolism in insulin-sensitive tissues such as liver and muscle. In the liver, adiponectin promotes glucose use and enhances fatty acid oxidation by improvement of insulin action via activation of AMPK [95,96]. In addition, adiponectin has an anti-inflammatory effect by neutralizing TNF-α, and improves hepatic steatosis and inflammation [97].
Anabolic hormones, such as GH and IGF-1, decline with aging process, which affects the progressive loss of muscle mass [98]. Fat accumulation and aging impair the GH/IGF-1 signaling pathway, leading to deterioration of muscle mass synthesis [99,100]. In an experiental mouse model of NAFLD, NAFLD was associated with decreased muscle mass and strength, and reduced IGF-1 level, implicating that IGF-1 reduction might play a role in the development of NAFLD-related sarcopenia [101].
BIDIRECTIONAL RELATIONSHIP BETWEEN SARCOPENIA AND NAFLD
Numerous studies have reported a relationship between NAFLD and sarcopenia (Tables 1, 2). Sarcopenia is a risk factor for the presence and severity of NAFLD (Table 1) [7,22,102,103]. The prevalence of sarcopenia is significantly increased in NAFLD and NASH compared to that in non-NAFLD (17.9% and 35.0% vs. 8.7%, respectively) [3]. NAFLD patients with sarcopenia had a 2-fold higher risk of developing NASH and significant fibrosis independent of obesity and IR [3]. However, most studies were cross-sectional in design and the causal relationship between sarcopenia and NAFLD remains unclear. A recent study demonstrated that NAFLD was developed in 14.8% of its participants during a 7-year follow-up, with an increased incidence in participants with the lowest tertile of skeletal muscle mass at baseline. Baseline skeletal muscle mass was also positively associated with the resolution of existing NAFLD, regardless of metabolic risk factors [10]. Sarcopenia was associated with poor clinical outcomes, including severe hepatic fibrosis and increased mortality, in NAFLD patients [104-106]. Hence, low skeletal muscle mass may cause the development of NAFLD. In a multicenter prospective study, hepatic steatosis at baseline was significantly associated with the risk of sarcopenia in older adults. Lower muscle mass and strength were more common in NAFLD patients [16]. In another study, the loss of skeletal muscle mass was faster in subjects with NAFLD compared to those without NAFLD. When stratified by fibrosis severity, skeletal muscle mass loss was faster in NAFLD subjects with an intermediate-to-high probability of advanced fibrosis than in those without (Table 2) [107].
Muscle quality also plays a critical role in the development of NASH. Myosteatosis determines muscle strength and function, and metabolic and liver-related clinical outcomes [108-110]. It is a prognosticator for NASH development [108,111,112]. Studies have suggested that myosteatosis is a clinically useful surrogate marker for NASH [108] by demonstrating that severe myosteatosis, but not sarcopenia, predicts NASH development and fibrosis progression [111]. The prevalence of myosteatosis is increased in obese subjects with NASH; hence, myosteatosis could reflect the histological features of NASH [110]. Muscle alterations are linked with fibrosis severity in subjects with NAFLD [3-5,9,22,113-117]. These suggest that the role of sarcopenia in NASH development is unclear. Both sarcopenia and myosteatosis have been linked to advanced fibrosis and cirrhosis [22,34,118-121]. However, the relatively low skeletal muscle mass observed in NAFLD patients may derive from increased body fat percentage [15,110]. Muscle wasting is often seen in patients with advanced fibrosis, implicating reverse causality between low skeletal muscle mass and NAFLD severity [9,14]. Patients with liver cirrhosis had concomitant sarcopenia (43%), sarcopenic obesity (low muscle mass with obesity) (26%), and myosteatosis (52%) [34]. Hence, advanced fibrosis is more likely to cause sarcopenia rather than sarcopenia causing fibrosis progression.
CONCLUSIONS
It is currently difficult to conclude whether sarcopenia is a risk factor or a consequence of NASH. However, sarcopenia and NAFLD are linked by several shared pathogenetic mechanisms, implicating a bidirectional relationship between sarcopenia and NAFLD. Therefore, further studies are needed to investigate the effects of low muscle function and performance on NAFLD progression. In addition, prospective standardized trials with accurate diagnoses of sarcopenia and NAFLD are warranted to elucidate the cause-and-effect relationship between sarcopenia and NAFLD.
Notes
Authors’ contribution
Sae Kyung Joo: drafting the manuscript; preparation of the figure and table. Won Kim: design of the work; supervision of the article; obtaining funding.
Conflicts of Interest
The authors have no conflicts to disclose.
Acknowledgements
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (2021R1A2C2005820 and 2021M3A9E4021818), the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (HI21C0538), and the Research Program funded by the Korea Centers for Disease Control and Prevention (2022ER090200).
Abbreviations
CRP
C-reactive protein
FFA
free fatty acid
GH
growth hormone
IGF-1
insulin growth factor-1
IL-6
interleukin-6
IR
insulin resistance
NAFLD
nonalcoholic fatty liver disease
NASH
nonalcoholic steatohepatitis
TG
triglyceride
T2DM
type 2 diabetes mellitus
TGF-β
transforming growth factor-β
TNF-α
tumor necrosis factor-α
VDR
vitamin D receptor