Long-term prognosis and the need for histologic assessment of chronic hepatitis B in the serological immune-tolerant phase
Article information
See the commentary-article "The imitator of immune-tolerant chronic hepatitis B: A killer in disguise" on page 363.
See the commentary-article "Is liver biopsy essential to identifying the immune tolerant phase of chronic hepatitis B?" on page 367.
Abstract
Background/Aims
The histologic status of the immune-tolerant (IT) phase of chronic hepatitis B relative to long-term outcomes is unclear. This study aimed to discover how the serological criteria currently in use correspond to histologic criteria in determining the IT phase and indication for liver biopsy.
Methods
Patients in the serological IT phase determined by positive hepatitis B e antigen, hepatitis B virus (HBV) DNA ≥106 IU/mL, and normal or minimally elevated alanine aminotransferase (ALT) ≤60 IU/L, who underwent liver biopsy at three different hospitals were included. The distribution of the histologic IT phase, defined as fibrosis of stage 1 or less and inflammation of grade 1 or less, was compared with that of the serological IT phase. The risk factors for the incidence of liver-related events, such as hepatocellular carcinoma, liver cirrhosis, liver transplantation, and death, were also analyzed.
Results
Eighty-two (31.7%) out of 259 clinically suspected IT phase patients belonged to the histologic IT phase. Age over 35, high AST, and low albumin were useful for ruling out the histologic IT phase. Risk factors predicting liver-related events were age and significant fibrosis stage. There was no significant difference in the proportion of histologic IT phase and clinical prognosis between normal ALT and mildly elevated ALT groups. However, even in patients with normal ALT, age was an important factor in predicting the presence of the histologic IT phase.
Conclusions
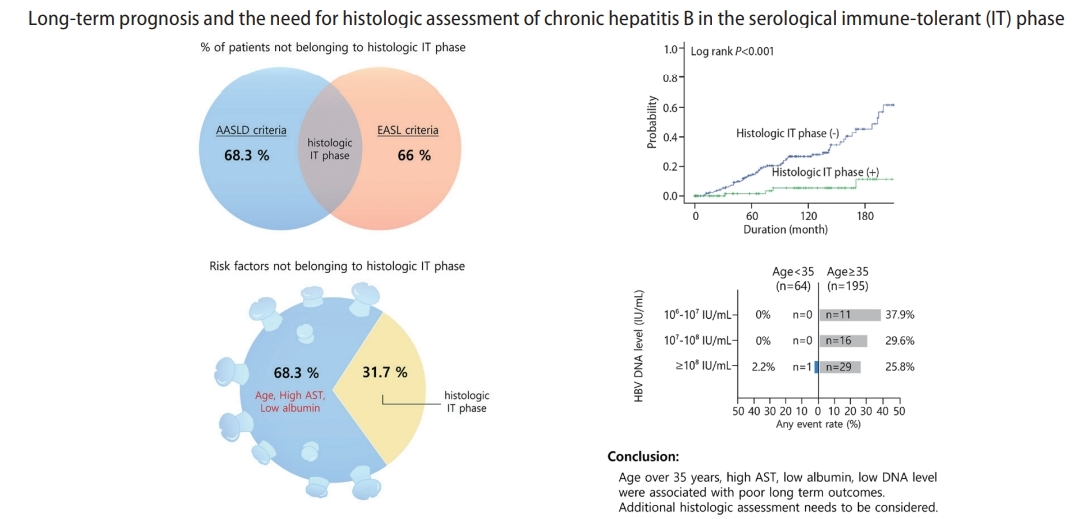
A significant number of patients who belonged to the serological IT phase were not in the histologic IT phase. Patients over 35 years and those with high AST, low albumin, and low HBV DNA levels were more likely to experience poor long-term clinical outcomes. Therefore, additional histologic assessment should be considered.
Graphical Abstract
INTRODUCTION
Advances in the knowledge of the evolution and phases of hepatitis B virus (HBV) acquired over the past 20 years, have allowed the gradual development of effective treatment options. As a result, the incidence of liver cirrhosis and liver cancer caused by hepatitis B has steadily decreased [1].
However, some aspects of HBV are not clearly understood, such as the so-called immune-tolerant (IT) phase, characterized by high HBV deoxyribonucleic acid (DNA) levels and yet, persistently normal alanine aminotransferase (ALT). The IT phase is associated with a good prognosis in general and thus, not recommended for antiviral treatment in most guidelines [2-5]. The limited understanding of the IT phase is evident in its arbitrary definition, which varies in different guidelines. For example, in the European Association for the Study of the Liver (EASL) guideline, the IT phase is defined as high levels of HBV DNA of more than 107 IU/mL [3], whereas in the American Association for the Study of Liver Diseases (AASLD) guideline, the definition is HBV DNA of more than 106 IU/mL [2]. Numerous studies have suggested that IT patients defined by serological criteria are no longer immunologically and histologically healthy, as supported by antigenspecific T-cell deletion, inadequate clonal expansion of effector T-cells, and consequently, functional tolerance evident as immune tolerance. However, HBV-specific T-cell responses, associated with clonal expansion of hepatocytes, can sometimes be detected in the early stages of HBV infection, especially in increased random integration of HBV DNA into infected hepatocytes. In addition, dysfunctional specific T-cells found in both IT and immune active phases suggest the ambiguity of boundaries that distinguish individual clinical phases.
Most classifications of the IT phase in recent guidelines are based on serum markers, especially HBV DNA and ALT and/or aspartate aminotransferase (AST). While such laboratory-based criteria are inevitable, as liver biopsies cannot be performed in all patients, it is well known that normal ALT and high DNA cannot conclusively rule out indolent fibrosis [6]. Therefore, clinicians have debated continuously whether treatment is necessary for patients in the IT phase.
Though a recent study published by Kim et al. [7], reported a notable finding of poorer prognosis in the IT phase than in the immune active phase with treatment, such findings may be limited because the subjects’ qualification as IT phase patients is in question [8]. Likewise, previous studies have attempted to define the IT phase based on serum markers, only to attain inconsistent results because definitions of the IT phase can vary by study. Therefore, it is essential to determine the definition of the IT phase and the criteria required for urgent treatment.
This study aimed to discover how well the serological criteria in current use correspond to the histologic criteria in determining the IT phase. In addition, we would like to suggest potential indications that require liver biopsy among patients in the serological IT phase.
MATERIALS AND METHODS
Patients and study protocol
We collected consecutively the data of 312 chronic hepatitis B patients in the clinical IT phase from January 1994 to December 2017. The patients underwent a liver biopsy to determine the progression and treatment of hepatitis. Patients who fulfilled the following inclusion criteria were eligible for this study: (a) patients over the age of 20, who underwent liver biopsy for chronic hepatitis B, (b) positive hepatitis B e antigen (HBeAg) and high HBV DNA in at least two tests taken more than six months apart, (c) normal or minimally elevated ALT (<60 U/L) in at least two tests taken more than three months apart, and (d) adequate histology in percutaneous liver biopsy. Patients were excluded for the following conditions: (a) HBV DNA lower than 106 IU/mL (n=26), (b) high AST or ALT (>60 IU/L) (n=18), (c) inadequate histology (n=2), (d) co-infection with chronic hepatitis C or hepatitis D (n=2) (e) features of chronic liver disease or liver cirrhosis in imaging studies (n=4), and (f) prior or current evidence of hepatocellular carcinoma (HCC) (n=1). We included 259 patients that met all criteria in the final selection (Supplementary Fig. 1). Clinical, histologic, and laboratory records of the involved patients were reviewed retrospectively.
The study protocol was approved by the Institutional Review Board of our hospital (IRB number SCHBC-2020-03-031-001, registration date: 7 April 2020). The study protocol conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki.
Liver biopsy and histology
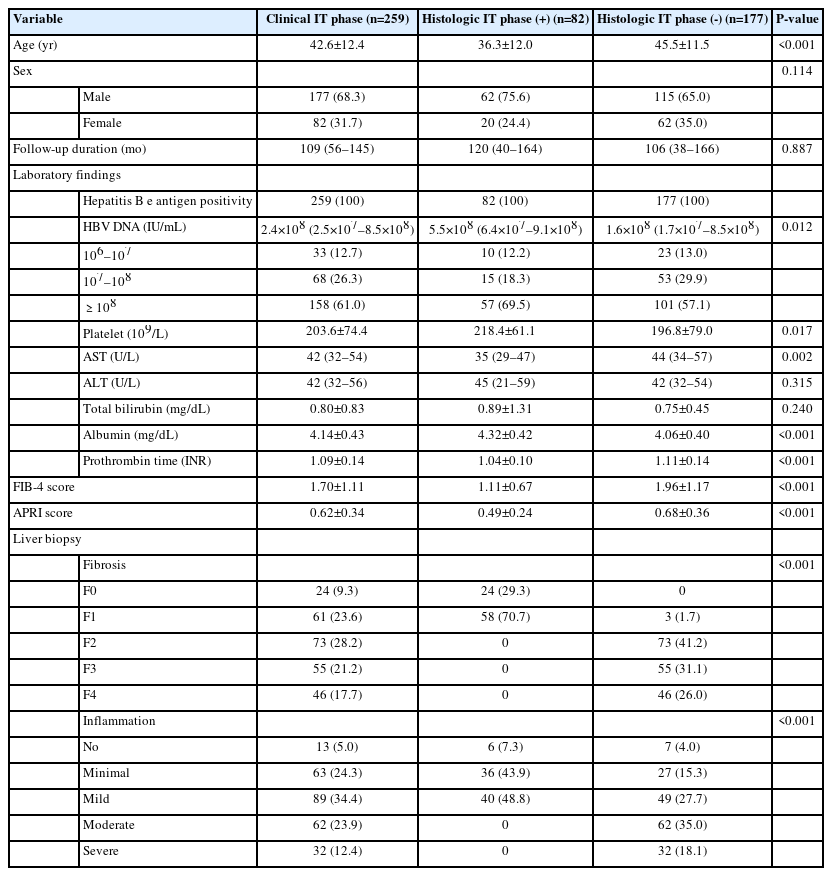
A liver biopsy was conducted when each investigator deemed it necessary to evaluate the status or severity of chronic hepatitis B, to determine the need for antiviral therapy, or to identify autoimmune diseases or metabolic diseases. Ultrasound-guided liver biopsy was performed by expert hepatologists experienced with over 500 ultrasound procedures and 100 liver biopsies. An adequate liver biopsy sample was characterized by a length of 2 to 3 cm or more and the inclusion of ten or more portal tracts [9,10]. Specimens were fixed in formalin and embedded in paraffin. The resulting sections were stained with hematoxylin-eosin and Masson’s trichrome. Each biopsy specimen was analyzed by pathologists from each institution with over ten years of experience. The inability to confirm the degree of agreement among pathologists is one of the major limitations of this retrospective study. Histologic grading and staging of the liver biopsy were described according to the standardized guideline proposed by the Korean Study Group for the Pathology of Digestive Disease [11]. Fibrosis was assessed on a scale of 0 to 4: F0, no fibrosis; F1, portal fibrosis without septa; F2, periportal fibrosis; F3, septal fibrosis; and F4, liver cirrhosis. Inflammation was graded as none (G0), minimal (G1), mild (G2), moderate (G3), or severe (G4). Specimens of at least 20 mm in length and with 11 or more portal tracts included were considered eligible for interpretation in this study [12].
Outcomes, definition, and follow-up
The primary goal of the study was to evaluate the compatibility between pre-existing serological criteria and histologic criteria in determining the IT phase. The secondary goal was to find long-term prognostic factors in IT phase patients in association with outcomes of interest, such as liver cirrhosis, HCC, liver transplantation (LT), or death.
The definition of the IT phase according to the AASLD is as follows: (a) positive hepatitis B surface antigen for more than six months, (b) positive hepatitis B e antigen, (c) HBV DNA level higher than one million IU/mL and (d) normal (35 U/L for males and 25 U/L for females) or minimally elevated ALT [2].
The definition of the IT phase according to the EASL is as follows: (a) positive hepatitis B surface antigen for more than six months, (b) positive hepatitis B e antigen, (c) HBV DNA level higher than 107 IU/mL, and (d) normal ALT (40 U/L) [3].
The definition of the histologic IT phase is as follows: fibrosis of stage 1 or less and inflammation of grade 1 or less.
Liver cirrhosis was diagnosed by the presence of diffuse nodular surface or regeneration, dense fibrous septa, and architectural or hepatic vascular distortion in follow-up imaging studies, such as ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) [13]. HCC was confirmed by the presence of typical features (arterial enhancement and portal-delayed washout in nodules of more than one centimeter) in imaging studies, including CT or MRI, or in histologic studies [14].
The index date was defined as the date of liver biopsy. The follow-up period was calculated from the index date to the date of the outcome of interest or the last follow-up date. Patients regularly attended the laboratory and/or abdominal ultrasound check-ups every three to six months. A liver-related event was defined as the occurrence of liver cirrhosis, HCC, LT, or death.
Statistical analysis
Frequencies and percentages were used for descriptive statistics. Statistical differences between groups were investigated using the χ2 test and Student’s t-test. Spearman’s analysis was used to investigate correlations between variables. The cumulative incidence of liver-related events between patients in and those not in the IT phase was estimated using the Kaplan-Meier method, and differences between the curves were compared using the log-rank test. Multivariable logistic regression analysis was used for risk factors to exclude patients in the histologic IT phase. Factors known to be effective in predicting the IT phase in previous studies (e.g., sex, age, body mass index, HBV DNA, platelet, AST, ALT, albumin, and total bilirubin) were analyzed. Cox proportional hazards model was used as the main analysis tool to calculate the incidence of liver-related events. Factors known to be associated with long-term liver-related events in previous studies (e.g., sex, age, HBV DNA, platelet, AST, ALT, albumin, total bilirubin, antiviral treatment, fibrosis stage, and inflammation grade) were analyzed. Multivariate models were created using variables that were clinically relevant and significant (P<0.10) in univariate analysis. All statistical analyses were performed using R (version 3.3.3, The R Foundation for Statistical Computing, Vienna, Austria) and SPSS software (ver. 21.0; SPSS Inc., Armonk, NY, USA). Statistical significance was defined as P<0.05.
RESULTS
Baseline characteristics
The baseline demographics and clinical characteristics of patients are summarized in Table 1. A total of 259 patients were analyzed, including 177 (68.3%) males. The patients were 42.7±12.5 years old on average. The median HBV DNA level was 2.4×108 IU/mL (interquartile range [IQR] 2.5×107– 8.5×108), and AST and ALT levels were 42 U/L (IQR 32–54) and 42 U/L (IQR 32–56), respectively. The median follow-up duration was 109 months (IQR 56–145).
The distribution of fibrosis stages and inflammation grades in patients is also presented in Table 1. Although all patients were not expected to exhibit advanced liver disease on imaging, advanced fibrosis (≥F3) was observed in as many as 101 (38.9%) patients. Similarly, given the low level of ALT, there was a significant number of patients (94, 36.3%) with inflammation more severe than the moderate grade.
Proportion of patients with histologic IT phase in comparison with the AASLD and EASL criteria
Among the 259 patients in the serological IT phase, 82 (31.7%) patients were in the histologic IT phase. Patients in the histologic IT phase were younger (36.39 years vs. 42.67 years) on average than those not in the histologic IT phase. The histologic IT phase patients also had comparatively higher HBV DNA (5.5×108 IU/mL vs. 2.4×108 IU/mL), lower AST (35 U/L vs. 42 U/L), higher albumin, and lower prothrombin time (PT) international normalized ratio (INR) levels (Table 1).
We evaluated the correlation between the current serological criteria provided by AASLD and EASL and the histologic criteria of the IT phase. Out of the enrolled patients, 259 and 100 patients met the serological criteria of the IT phase provided by AASLD and EASL, respectively. Among the 259 patients who satisfied the AASLD criteria, 82 patients (31.7%) were identified to be in the histologic IT phase. Among the 100 patients who satisfied the EASL criteria, 34 patients (34.0%) were identified to be in the histologic IT phase (Supplementary Fig. 2A). In summary, 68.3% and 66.0% of patients adhering to the serological IT phase criteria provided by AASLD and EASL, respectively, were not in the IT phase histologically and may have been in the immune clearance phase.
Similarly, for those patients with ALT within normal limits (≤25 IU for women and ≤35 IU for men), 64.5% and 64.9% of patients adhering to serological IT phase criteria provided by AASLD and EASL, respectively, were not in the IT phase histologically (Supplementary Fig. 2B).
Clinical parameters that can predict the patients who are not likely to be in the histologic IT phase
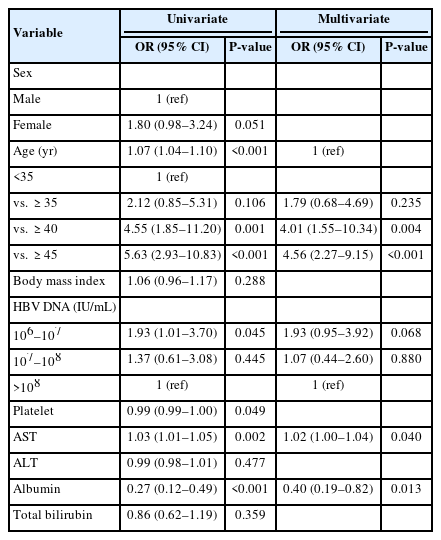
We investigated useful clinical parameters that can predict patients who are not likely to truly be in the histologic IT phase (Table 2). In a multivariate analysis, factors such as age over 35 years (odds ratio [OR] 1.48, 95% confidence interval [CI] 0.45–4.81, P=0.005), high AST (OR 1.03, 95% CI 1.01–1.06; P=0.015) and low albumin level (OR 0.28, 95% CI 0.11–0.73; P=0.010) were useful indicators for ruling out histologic IT phase. On the other hand, high HBV DNA (≥108 IU/mL) or gender were not significant factors in predicting the histologic IT phase.

Clinical indicators that can predict patients who are not likely to be in the histologic immune-tolerant phase
Similarly, we searched for clinical indicators that can predict significant fibrosis (≥F2). Age over 35 years, high AST, and low albumin were significant indicators and were also useful for ruling out the histologic IT phase (Table 3). Though not statistically significant, patients with low HBV DNA levels (106–107 IU/mL) had a higher probability of significant fibrosis than patients with high HBV DNA levels.
Factors related with the incidence of liver-related events (liver cirrhosis, HCC, LT, or death)
During the observation period of 109 months, 192 patients (74.1%) switched to the immune-active phase and started antiviral therapy. The average time to transition to the immune-active phase was 40.1±48.4 months. During this period, the development of liver cirrhosis and HCC was evident in 42 (16.2%) and 17 (6.6%) patients, respectively. Events such as LT and death also occurred in one (0.4%) and 21 patients (8.1%), respectively. The prediction of long-term prognosis was compared between the two IT phase classification guidelines. For patients in the histologic IT phase, the incidence of liver-related events was significantly lower than that of patients not in the histologic IT phase (log-rank P<0.001, Fig. 1A).
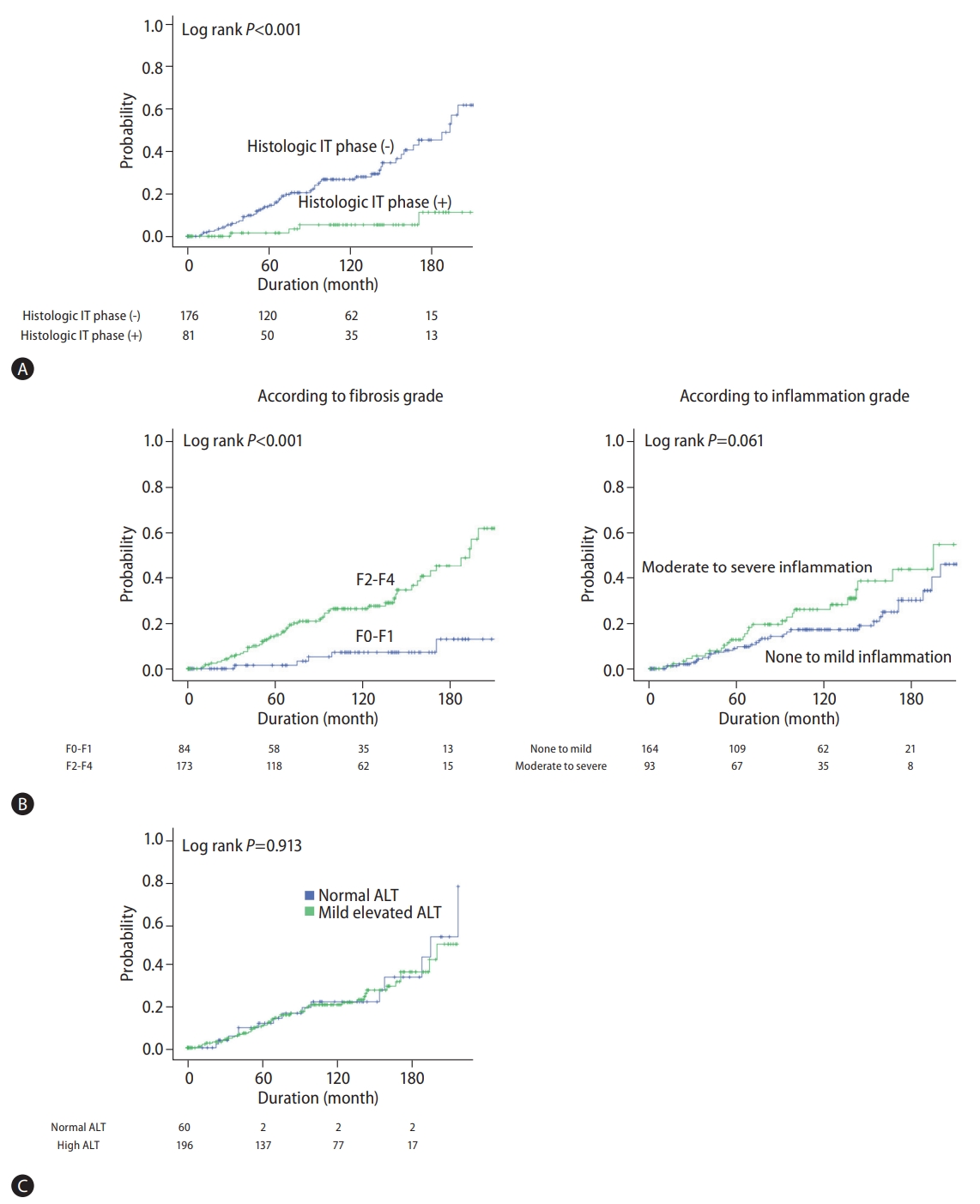
Kaplan-Meier curves showing the cumulative incidence of liver-related events (liver cirrhosis, hepatocellular carcinoma, liver transplantation, or death). (A) According to histologic IT phase, (B) according to fibrosis and inflammation grade, (C) according to ALT level. IT, immune-tolerant; ALT, alanine aminotransferase.
We further conducted the Cox regression analysis to identify factors related to the incidence of liver-related events (liver cirrhosis, HCC, LT, or death). According to our multivariate analysis, age (hazard ratio [HR] 1.077, 95% CI 1.045–1.110; P<0.0001) and significant fibrosis (F2-F4) (HR 3.650, 95% CI 1.375–9.694; P=0.009) were closely related to the occurrence of liver-related events. On the other hand, histologic inflammation did not show any association with the occurrence of liver-related events (Table 4). Similarly, in the Kaplan-Meier analysis, the fibrosis stage was associated with the occurrence of liver-related events, whereas inflammation grade showed no evidence of such an association (Fig. 1B).
Further analysis was performed by limiting liver-related events to ‘HCC, LT, and death’, demonstrating similar results and patterns. Age and significant fibrosis were significantly related to the occurrence of HCC, LT, and death. Results of the Kaplan-Meier analysis and Cox regression of sensitivity analysis are presented in Supplementary Table 1 and Supplementary Figure 3.
Importance of age and HBV DNA level for prediction of liver-related events
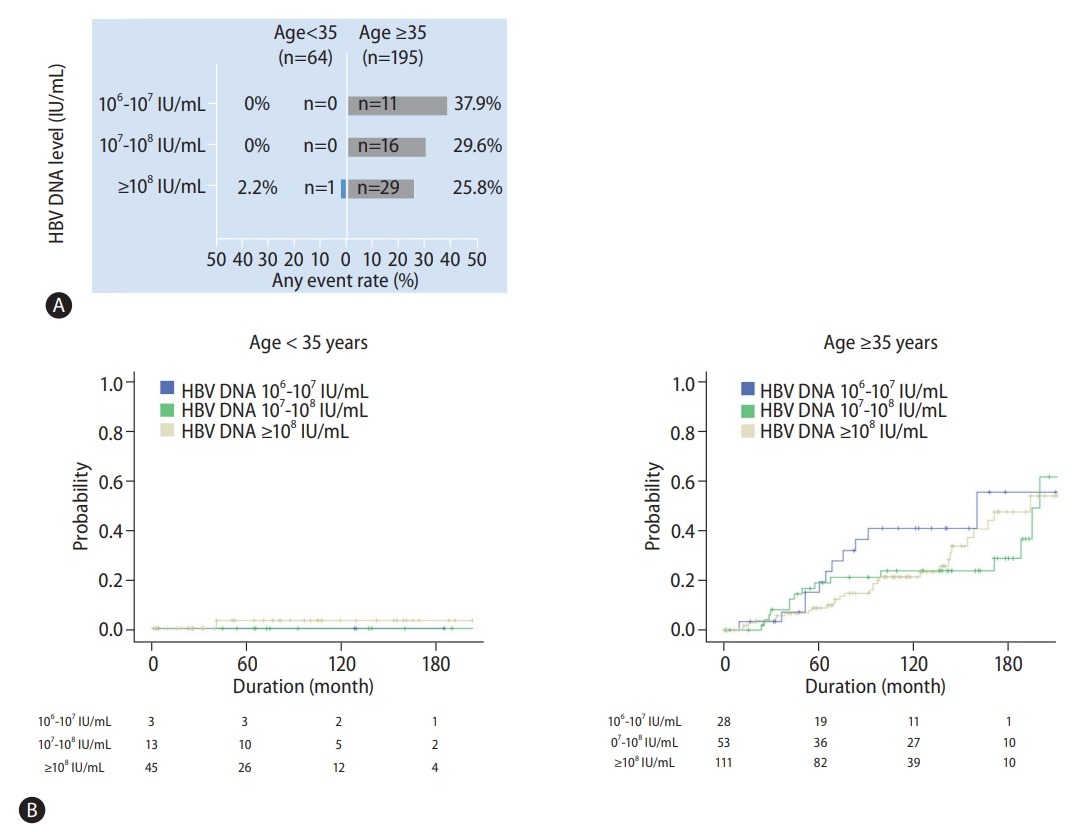
Various guidelines have mostly used patient age and HBV DNA level to identify the clinical IT phase. We performed a stratified analysis to determine the rate of liver-related events according to age and HBV DNA level (Fig. 2A). Patients under the age of 35 exhibited a very low occurrence rate of liver-related events, regardless of the HBV DNA level (n=1, 1.6%). The incidence of liver-related events was significantly higher in patients over the age of 35 compared to that in patients under the age of 35 (28.7% vs. 1.6%). In particular, the incidence of liver-related events tended to increase (HBV DNA ≥108 IU/mL, 25.8%; DNA 107–108 IU/mL, 29.6%; DNA 106–107 IU/mL, 37.9%) as the HBV DNA level decreased. Such a trend was further confirmed through the Kaplan-Meier analysis (Fig. 2B).
IT phase patients with normal ALT
We performed a subgroup analysis on patients with normal ALT (≤25 IU for women and ≤35 IU for men). According to histological indication, there was no significant difference in the proportion of patients eligible for treatment between normal ALT and mildly elevated ALT groups (64.5% vs. 69.5%, P=0.531). In addition, the clinical prognosis of the patients with normal ALT eligible for treatment was not as good as that of patients with high ALT (Fig. 1C; log-rank P=0.913). On the other hand, even in patients with normal ALT, age was an important factor in predicting the presence of the histologic IT phase. Patients aged 35 or older were significantly more likely to be eligible for antiviral treatment than those under 35 years of age (75.0% vs. 45.0%, P=0.028).
DISCUSSION
Our study demonstrates that the serologically defined IT phase used currently is more inconsistent with the histologic IT phase based on liver biopsy than expected. We also identified additional clinical parameters closely related to the histologic IT phase aside from the previously known HBV DNA, AST, and ALT. This study highlights the necessity for the careful evaluation of the IT profile in adults, the impact of age in predicting fibrosis of F2 or higher, and the importance of liver biopsy.
Ever since the IT phase was first conceptualized by Professor Chu in 1985 [15], it has been classified as a benign phase due to the absence of histologic progression observed during follow-up. However, an increasing number of studies with immunological perspectives have questioned such a notion. A study by Mason et al. [16] found that HBV-specific T cells in the IT phase did not differ from those in the immune active phase. In addition, host genome integration, which is considered the first step in promoting HCC, has already been discovered in the IT phase [17]. According to a similar study involving asymptomatic hepatitis B surface antigen (HBsAg) carrier children from four to nine years old, liver biopsy showed definite histologic changes in the livers of all subjects [18]. In addition to this immunological evidence, clinical findings yield corroborating results of higher HCC incidence in the IT phase than that in the treated immune active phase [7]. Some researchers even argued that the term “IT phase” is a misnomer, suggesting the phrase “high replicative, low inflammatory” as a substitution. Similarly, the EASL guideline changed the term “IT phase” to “HBeAg positive infection” [3,16].
The definition of the IT phase, even the legitimacy of its very existence, has remained debatable [19]. However, it is crucial to evaluate its definition. Without a rigorous classification of the IT phase, any following conclusion will forever remain invalid. The problem with the IT phase defined by current guidelines, which use clinical and virological parameters [2,3]. is the overestimation of the incidence of the true IT phase. Moreover, under the current guidelines, it is not possible to distinguish the true histologic IT phase and delayed HBeAg seroconversion [8]. It is essential to distinguish the two clinically, since the true histologic IT phase demonstrates a good prognosis, whereas delayed HBeAg seroconversion leads to increased risks of HCC and liver cirrhosis. The long-term outcome including HCC in the IT phase has remained debatable among the studies [7,20,21]. Most studies have defined the IT phase based on HBV DNA and ALT levels. Previous studies, with the use of pre-existing definitions, are likely to have unintentionally included immune clearance stage patients or delayed HBeAg seroconversion patients, leading to unreliable population samples. Similarly, the sensitivity values of AASLD and EASL criteria were low in our analysis, 16 and 33%, respectively, indicating pre-existing diagnostic criteria cannot accurately identify “true IT phase patients.”
The first finding of our study was in identifying effective indicators that can predict the development of liver-related events, such as liver cirrhosis, HCC, LT, and death [22,23]. Our study found that it is impossible to determine the prognosis using serological criteria of HBV DNA and ALT. In fact, there have been studies reporting a good prognosis in the biomarker-defined IT phase, but these studies additionally included conditions, such as low Fibrosis-4 (FIB-4) score or age<40 years, in addition to the existing HBV DNA and ALT levels [24-26]. In the serological IT phase defined by HBV DNA and ALT, one study reported that only 50.3% of patients remained in the IT phase throughout the study period of 63 months [27]. We concluded that liver biopsy was the only accurate method of evaluating histologic liver fibrosis in IT phase patients. Additionally, histologic fibrosis was associated with long-term prognosis in our analysis. The occurrence rate of liver-related events was 3.65 times higher in the fibrosis of F2 or higher than that in fibrosis of F0 or F1. Therefore, for patients in the suspected IT phase, it is advisable to consider the fibrosis stage regardless of virological markers. Instead of the liver biopsy, transient elastography may be an alternative tool to assess the fibrosis stage, though a potential shortcoming lies in its inability to distinguish fibrosis and moderate-to-severe necroinflammation [28]. Furthermore, the liver stiffness value has been reported to be affected by the degree of inflammation even at a low ALT level [29]. Our analysis of FIB-4 and AST to Platelet Ratio Index (APRI) substantiates such findings, as the proportions of advanced fibrosis (≥F3) in low FIB-4 (<1.45) and in low APRI (<1.0) were 18 and 29%, respectively. Therefore, the clinical usefulness of FIB-4 or APRI is notably low for patients in the IT phase.
The secondary finding of our study was in determining whether a biopsy is necessary for all patients with suspected IT phase. Because liver biopsy, due to its invasive nature, cannot practically be conducted in all patients, it is preferable to conduct biopsy only in patients with advanced fibrosis, the most relevant predictor of long-term prognosis. To reduce the usage of such invasive diagnostic methods, we analyzed clinical factors that can predict fibrosis of F2 or higher. Our results demonstrated that the probability of fibrosis F2 or higher increased significantly by 1.3 times in patients over 35 years, indicating age was the only clinical predictor of fibrosis. Contrary to previous studies, in which age was not considered clinically crucial compared to ALT and HBV DNA, our study suggests otherwise: liver biopsy might be recommended for patients over 35 years of age to evaluate the fibrosis stage histologically.
The tertiary finding of our study is the need for antiviral therapy in the IT phase. Clinical practices recommended by current guidelines cannot accurately identify those who need HBV suppression for HCC prevention among IT phase patients. With the exclusion of such patients receiving treatment based on current guidelines, a missed opportunity to prevent future liver complications inevitably follows. We believe antiviral treatment targeted to the appropriate group will not only hinder liver cirrhosis progression but also prevent HCC. However, it is difficult to use antiviral therapy in every IT phase patient, considering its cost-effectiveness, low adherence in younger patients, long-term side effects, and low virological response in the IT phase [30]. Therefore, to determine the necessity of antiviral therapy, liver biopsy is necessary for patients who have sufficient risk factors for histologic fibrosis of F2 or higher, considering their poor long-term prognosis.
Finally, it is notable that liver-related events tended to increase as HBV DNA decreased in IT phase patients aged over 35 years. Although the strong positive correlation between HBV DNA level and liver-related events such as HCC in hepatitis B patients is well known, an exception is made in IT phase patients, because HBV DNA usually remains very high— above 107 IU/mL—in patients who have never undergone immune clearance [19]. Therefore, a gradual decrease in HBV DNA level in patients with the IT phase suggests a possibility of immune clearance, necessitating a close clinical observation.
The most important limitation of our study is that our results are derived from retrospective cohort data. First, the time span of our study is too long. During the study period, the diagnostic criteria and guidelines for chronic hepatitis B have been updated and adjusted numerous times; thus, a risk of selection bias remains. In addition, it was not possible to monitor liver-related events according to the specific protocol in all patients. However, ultrasounds, CT scans, or laboratory tests were performed regularly every six months according to the practice guidelines. Also, mortality data in patients with LT were confirmed by the Korean Statistics Promotion Institute (http://stat.or.kr/) and the registry of the Korean Network for Organ Sharing, respectively. Secondly, most Korean hepatitis B patients are known to have genotype C, which displays a delayed e-antigen seroconversion [31]. Therefore, it is difficult to generalize the 35-year cut-off to other genotypes. A random sampling error in biopsy may exist in our study. Finally, the previous serological criteria were used to include all patients who were presumably in the IT phase, thus we found more patients with advanced liver disease than previously expected. Therefore, we believe that some of the patients might be in the stage of regression of flare in ‘HBeAg positive, immune active infection.’ Regardless, it was helpful to see how many patients in these diverse spectra actually were in the histological IT phase and to further refine the indications for liver biopsy. In addition, similar results were found in subgroup analysis in patients with normal ALT (≤25 IU for women and ≤35 IU for men).
Future studies on patients in the suspected IT phase need to be conducted to predict the prognosis, using other non-invasive methods to determine fibrosis. Furthermore, the effect of antiviral treatment on the long-term prognosis in patients diagnosed with advanced fibrosis (F2 or higher) through non-invasive methods must be thoroughly scrutinized.
In conclusion, for IT phase patients aged 35 or older who are contemplating treatment options, liver biopsy should be considered without delay instead of waiting for an increase in ALT levels.
Notes
Authors’ contribution
Study concept and design: Sang Gyune Kim and Yeon Seok Seo. Formal Analysis: Jeong-Ju Yoo. Investigation: All authors. Manuscript writing: Jeong-Ju Yoo. Manuscript review & editing: Sang Gyune Kim and Yeon Seok Seo. Final approval of manuscript: All authors.
Conflicts of Interest
The authors have no conflictsto disclose.
Acknowledgements
We would like to Jae-Young Kim in Research Factory Inc. (www.rfactory.co.kr) for consulting the statistical analysis.
This research was funded by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (2021R1G1A1007886), and in part by the Soonchunhyang University Re-search Fund.
Supplementary materials
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
Time-dependent covariate Cox regression analysis predicting liver related event
Flow chart of the patients. HBV, hepatitis B virus; ALT, alanine aminotransferase; AST, aspartate aminotransferase; IT, immune-tolerant.
Proportion of immune-tolerant phase according to each diagnostic criterion. (A) Total, (B) ALT within normal limits. ALT, alanine aminotransferase; AASLD, American Association for the Study of Liver Diseases; EASL, European Association for the Study of the Liver; IT, immune-tolerant.
Kaplan-Meier curves showing the cumulative incidence of liver-related events (hepatocellular carcinoma, liver transplantation, or death). (A) According to the histologic IT phase, (B) according to fibrosis and inflammation grade. IT, immune-tolerant.
Abbreviations
AASLD
American Association for the Study of Liver Diseases
ALT
alanine aminotransferase
AST
aspartate aminotransferase
CI
confidence interval
EASL
European Association for the Study of the Liver
HBV
hepatitis B virus
HCC
Hepatocellular carcinoma
HR
hazard ratio
IRB
Institutional Review Board of our hospital
IT
immunetolerant
OR
odds ratio
References
Article information Continued
Notes
Study Highlights
• Previous studies have attempted to define IT phase of chronic hepatitis B based on serum markers, only to attain inconsistent results, due to definitions of IT phase varying by studies. Therefore, it is essential to determine the definition of IT phase and criteria required for urgent treatment. Eighty-two (31.7%) out of 259 clinically suspected IT phase patients belonged to histologic IT phase. Among patients in IT phase identified by the AASLD and EASL criteria, 31.7% and 34.0% were in IT phase histologically, respectively. Old age, high AST and low albumin were useful for ruling out histologic IT phase. In conclusion, numerous patients in clinically suspected IT phase were not in IT phase histologically. Liver biopsy should be recommended to determine treatment for such patients.