The role of different viral biomarkers on the management of chronic hepatitis B
Article information
Abstract
Chronic hepatitis B infection is a major public health challenge. With the advancement in technology, various components of the viral cycle can now be measured in the blood to assess viral activity. In this review article, we summarize the relevant data of how antiviral therapies impact viral biomarkers, and discuss their potential implications. Viral nucleic acids including hepatitis B virus (HBV) double-stranded deoxy-ribonucleic acid (DNA) and to a lesser extent, pre-genomic RNA, are readily suppressed by nucleos(t)ide analogues (NUCs). The primary role of these markers include risk prediction for hepatocellular carcinoma (HCC) and risk stratification for partial cure, defined as off-therapy virological control, or functional cure, defined as hepatitis B surface antigen (HBsAg) seroclearance plus undetectable serum HBV DNA for ≥6 months. Viral translational products including hepatitis e antigen, quantitative HBsAg and hepatitis B core-related antigen can be reduced by NUCs and pegylated interferon a. They are important in defining disease phase, delineating treatment endpoints, and predicting clinical outcomes including HCC risk and partial/functional cure. As the primary outcome of phase III trials in chronic hepatitis B is set as HBsAg seroclearance, appropriate viral biomarkers can potentially inform the efficacy of novel compounds. Early viral biomarker response can help with prioritization of subjects into clinical trials. However, standardization and validation studies would be crucial before viral biomarkers can be broadly implemented in clinical use.
DISEASE BURDEN OF CHRONIC HEPATITIS B INFECTION
Chronic hepatitis B (CHB) infection affects 292 million people globally, and is a major cause of liver-related morbidities including liver failure, cirrhosis and hepatocellular carcinoma (HCC) [1]. As a significant public health concern, the World Health Organization (WHO) has set goals to reduce the incidence of CHB and associated mortality by year 2030 [2]. The majority of people with CHB acquired the infection perinatally and during early childhood [3] when the immune system is not well equipped to mount a sufficient response against hepatitis B virus (HBV), which then establishes chronicity and becomes a lifelong infection in the vast majority of cases. Without treatment, up to 15–40% CHB subjects will progress to develop the liver-related morbidities. The adverse clinical events could be reduced by approved antiviral therapy which primarily acts by suppression of viral replication or immunomodulation. These mechanisms are being further explored to look for novel drug candidates to treat CHB infection. In addition, newer viral biomarkers have been identified to help evaluate treatment response and determine prognosis among treated patients. In this review, we will discuss the profile and potential applications of various blood-based viral biomarkers in CHB patients receiving antiviral therapy.
THE HBV VIRAL CYCLE
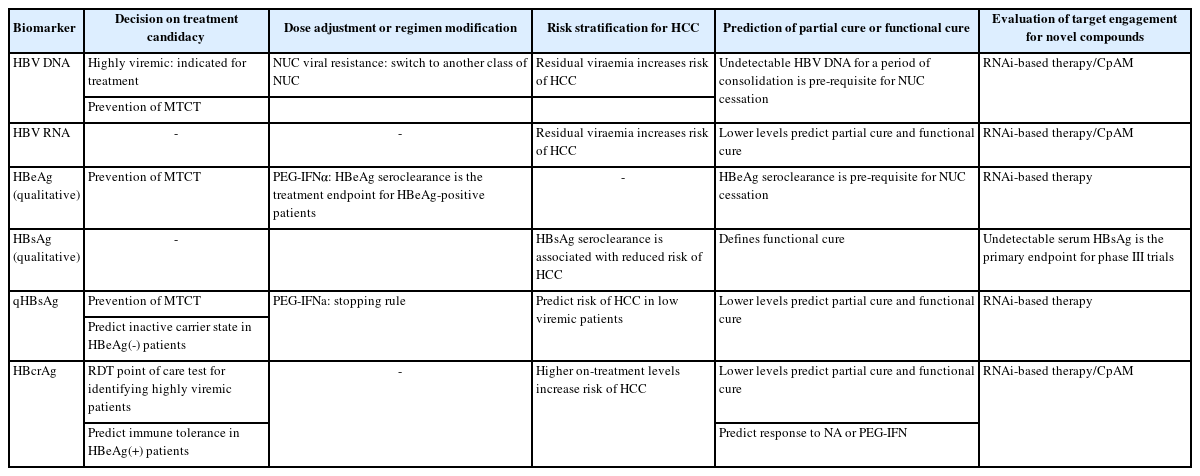
To date, there are no effective treatments to clear HBV from the infected liver due to the peculiar mechanisms of the viral cycle (Fig. 1). HBV is an enveloped, hepatotropic partially double-stranded deoxy-ribonucleic acid (DNA) virus. A mature HBV virion is fully encapsidated and contains relaxed circular (rc) DNA of approximately 3.2 kilobase pairs. Following entry into the hepatocytes via interaction with the sodium taurocholate co-transporting polypeptide [4], the rcDNA is imported to the nucleus [5] and is repaired by host cell DNA repair machinery [6,7] and converted to covalently closed circular DNA (cccDNA), which serves as the template for viral transcription [8]. The HBV genome consists of four overlapping open reading frames, which give rise to viral transcripts that include the pre-genomic RNA (pgRNA) and messenger RNAs for subsequent translation of viral proteins: hepatitis B surface antigen (HBsAg), hepatitis B e antigen (HBeAg), hepatitis B core antigen (HBcAg), X protein (HBx), and HBV polymerase. The pgRNA is packaged in a capsid made from HBcAg, a process also known as encapsidation, followed by reverse transcription into rcDNA and to a lesser extent, double-stranded linear DNA (dslDNA). These viral genomes are then envel-oped and released as infectious virions. The encapsidated rcDNA can be redirected to the nucleus to replenish the intranuclear cccDNA pool [9,10]. The persistence of cccDNA pool in the hepatocytes is the primary reason why it is not possible to eradicate the virus. A minority of mature HBV virions contains dslDNA, which are replication-deficient but are capable of host genome integration at sites of chromosomal DNA breaks. These form stable templates for synthesis of HBsAg and HBx [11], and can become potentially carcinogenic [12].
Viral cycle of hepatitis B virus. Those highlighted in asterisks are detectable in the bloodstream and can be used as viral biomarkers. These include HBsAg, HBeAg, HBcrAg, HBV DNA and pgRNA. cccDNA, covalently closed circular DNA; dslDNA, double-stranded linear DNA; HBV, hepatitis B virus; HBcAg, hepatitis core antigen; HBcrAg, hepatitis B core related antigen; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; mRNA, messenger RNA; NTCP, sodium taurocholate co-transporting polypeptide; pgRNA, pre-genomic RNA; rcDNA, relaxed circular DNA. *Detectable in the bloodstream.
TYPES OF ANTIVIRAL TREATMENT
There are two types of approved antiviral therapy in CHB: nucleos(t)ide analogues (NUCs) and pegylated interferona (PEG-IFNa). NUCs are DNA polymerase inhibitors that target the step of reverse transcription. As only a single step of the viral replication cycle is inhibited, there is relatively limited effects on the upstream events. The degree of viral suppression is limited to DNA synthesis, whereas cccDNA remains largely unaffected, and it would take a long time for the latter to decline. Therefore, NUCs need to be taken on a long-term basis, as premature withdrawal is associated with high rates of virological rebound [13,14]. The current first-line NUCs include entecavir, tenofovir disoproxil fumarate (TDF) and tenofovir alafenamide, all of which have a high barrier to viral resistance, and are generally well-tolerated.
The mechanisms of action for PEG-IFNa are less well-defined, but is believed to exert both immunomodulatory functions and direct antiviral properties. IFNa treatment induces a non-cytolytic antiviral state in the hepatocytes via regulation of gene expression and protein translation. One of the key mechanisms involve upregulation of APOBEC3 (a cytidine deaminase) which induces G-to-A hypermutations in the HBV genome and thereby inhibits viral replication [15] or even cccDNA degradation [16]. Also, IFNa treatment leads to cccDNAbound histone hypoacetylation and decreased binding to STAT1/STAT2 transcription factors, leading to reduced transcription of pgRNA from the cccDNA template [17]. Although PEG-IFNa can be given for a finite period (48-week course) as opposed to NUCs, HBV DNA suppression was suboptimal. In addition, it is administered subcutaneously and associated with numerous side effects, rendering it a less utilized treatment option in CHB.
SERUM VIRAL MARKERS TO EVALUATE TREATMENT RESPONSE
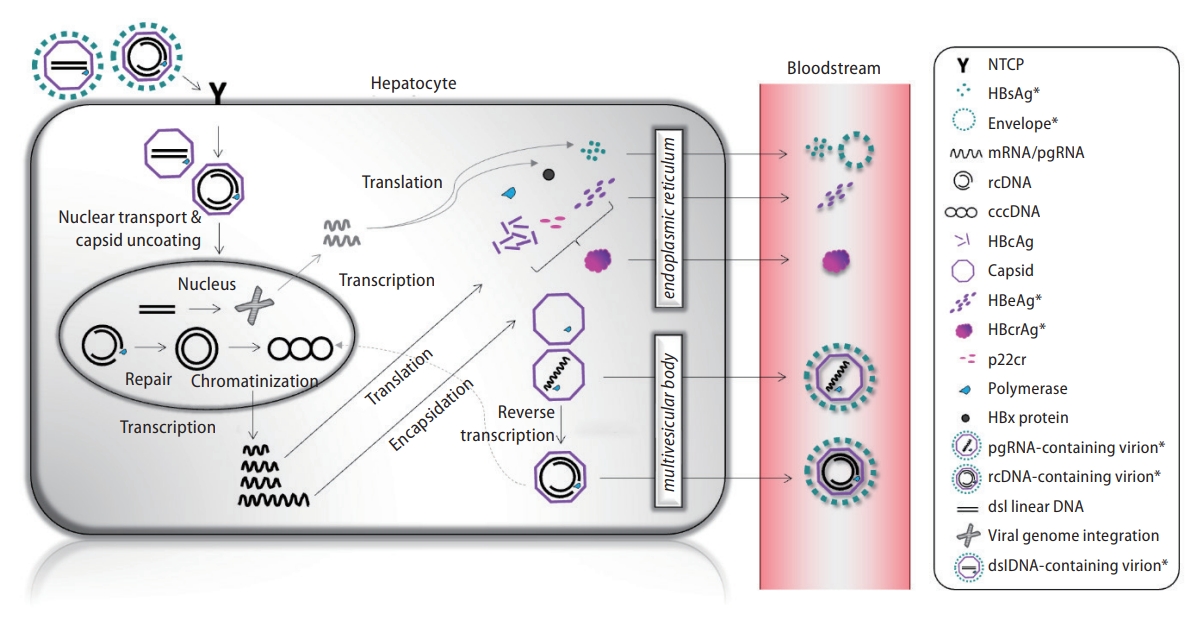
To assess treatment response, a number of viral biomarkers can be measured in the blood as a surrogate of the ongoing viral replicatory activities (Fig. 1). Well established markers such as HBV DNA and HBsAg have been incorporated as treatment endpoints in the CHB cascade of care (Fig. 2). On-treatment virological suppression, also known as incomplete cure, is the most reachable endpoint and can be achieved in >90% of NUC-treated subjects. Partial cure is defined as off-therapy virological suppression without HBsAg seroclearance, which is observed in around 20% subjects who received a finite course of therapy. Functional cure refers to sustained HBsAg seroclearance plus ≥6 months undetectable HBV DNA, which is associated with improved clinical outcomes but is only achieved by ~1% antiviral-treated subjects annually. Complete cure is defined as eradication of cccDNA, and sterilizing cure is defined as clearance of integrated DNA; both of which are unreachable with the current treatments. With these considerations, functional cure is regarded as the desirable treatment endpoint and has become a benchmark for phase 3 clinical trials of novel CHB therapy, with a threshold of HBsAg loss ≥30% as an arbitrarily acceptable rate of response 6 months after cessation of investigational compounds [18].
Treatment endpoints in the cascade of cure in chronic hepatitis B infection. cccDNA, covalently closed circular DNA; HBsAg, hepatitis B surface antigen; mRNA, messenger RNA; pgRNA, pre-genomic RNA; rcDNA, relaxed circular DNA; HBV, hepatitis B virus; DNA, double-stranded deoxy-ribonucleic acid.
The widespread use of blood-based viral biomarker stems from the need to quantify transcriptionally active intrahepatic cccDNA, which requires tissue specimens obtained from liver biopsy. Due to the invasive nature of the procedure, together with concerns from sampling error, intra/inter-observer variability and lack of standardization of the measurement, cccDNA quantification has largely remained as a research tool [19,20]. To this end, a number of blood-based HBV biomarkers has been studied as surrogate markers for cccDNA. They can be broadly classified as viral nucleic acids and translational products of HBV.
Viral nucleic acids
HBV DNA
The vast majority of detectable serum circulating HBV DNA is in the form of enveloped/encapsidated rcDNA [21]. In untreated patients, it shows moderate to good correlation with intrahepatic cccDNA (correlation coefficient r 0.36–0.49) [22-25]. The widely used in vitro nucleic acid amplification method allows high sensitivity of DNA detection and quantification, with lower limits reaching or below 1 to 2 log.
Upon NUC therapy, serum HBV DNA declines rapidly to undetectable levels. When assessed at 48 weeks, first-line NUC leads to undetectable serum HBV DNA in 64–76% and 90–94% of HBeAg-positive and HBeAg-negative patients, respectively [26]. For PEG-IFNa, after the complete course of 48 weeks, HBV DNA undetectability can be achieved in only 14% and 19% HBeAg-positive and HBeAg-negative patients, respectively [26].
HBV pgRNA
Circulating HBV RNA are encapsidated pgRNA in virus-like particles [27]. In untreated patients, it shows good to excellent correlation with intrahepatic cccDNA (r=0.59–89) [28-30]. Serum pgRNA can be measured with rapid amplification of complimentary DNA ends-based real-time polymerase chain reaction method [27,31], and the performance of RNA assays has been improved recently to approach the WHO standards [32]. Prior to antiviral treatment, serum HBV pgRNA levels are always 1–2 log lower than serum HBV DNA.
After a period of NUC treatment, the serum HBV RNA levels were decreased to a lesser extent than HBV DNA, leading to a reverse in serum RNA:DNA ratio [33]. Like serum HBV DNA, the correlation with cccDNA will be lost after antiviral therapy. The 48-week decline in HBV RNA was 1.46 log upon NUC treatment [33]. When assessed at 48 weeks of PEG-IFNa therapy among HBeAg-positive patients, the mean HBV RNA declined from 7.73 to 4.66 log [34]. For HBeAg-negative patients, upon PEG-IFNa and assessed at 48 weeks of therapy, a 1.72 log decline was observed from a baseline mean level of 4.4 log [35]. Unlike serum HBV DNA, the current use of HBV pgRNA measurement remains in the research context with no widely accepted standard to facilitate implementation in clinical use, and few comparisons of the various assays have been performed so far [32,33].
Translational products
HBeAg
The qualitative HBeAg has been more clinically relevant, being used to stratify disease phase and as an endpoint of treatment among HBeAg-positive patients (i.e., HBeAg seroclearance or seroconversion). In contrast, the quantitative HBeAg levels are mainly for research purpose, which can be quantified and expressed in Paul-Ehrlich Institute unit per mL (PEI-U/mL).
For HBeAg-positive patients treated with 48 weeks of NUC and PEG-IFNa, 10–21% and 32% achieved HBeAg seroclearance respectively [26]. Quantitative HBeAg levels correlated with serum HBV RNA (r=0.68), DNA (r=0.35), qHBsAg (r=0.20) and HBcrAg (r=0.69) [33]. In patients (predominantly genotype B/C) treated with PEG-IFNa, HBeAg levels declined starting from week 12 of therapy only in patients who achieved subsequent HBeAg seroconversion. HBeAg less than 17.55 PEI-U/mL at week 12 had positive predictive value and negative predictive value of 38% and 95% to predict HBeAg seroconversion at week 48 [36]. In another study involving CHB patients of Chinese ethnicity, baseline HBeAg levels were incorporated into a risk score which also include other biochemical variables (alanine aminotransferase, globulin and gamma-glutamyl transpeptidase) with a C-index of 0.776 to predict HBeAg seroconversion at 1 year [37].
HBsAg
The majority of HBsAg detected in the serum are subviral particles (SVP), which exceed mature virions by 100–100,000 times [38]. Commercially available assays can detect HBsAg in all forms (SVP or as part of the mature virion). HBsAg can be produced from either cccDNA or integrated DNA [39], with the latter contributing more in HBeAg negative patients. While the qualitative HBsAg informs whether treatment endpoint (functional cure) has been reached, quantitative HBsAg (qHBsAg) allows risk prediction for various clinical outcomes (see below). The lower limit of detection is around 0.05 IU/mL for most commonly used quantitative assays [40-42]. The clinical significance of measuring HBsAg by higher sensitivity assays with the lower limit of detection of 0.005 IU/mL and 0.0005 IU/mL [43-46] remains to be defined. Serum qHBsAg shows moderate to good correlation with intrahepatic cccDNA in untreated patients (correlation coefficient 0.28–0.71) [24,47] depending on the HBeAg status.
HBsAg seroclearance rate at 48 weeks of NUC treatment is 0–1% [26,48], although the event rate will slowly increase upon long-term treatment to <2% per year [48-53]. For PEG-IFNa recipients, the 1-year HBsAg seroclearance rate is 4% [26] and slowly increases with time after treatment completion (2.4% in 6.1 years) [54].
For patients treated with NUCs, the annual decline of qHBsAg was only 0.107 log [51], with only 16.1% patients achieving ≥1 log decline from baseline at 1 year of TDF therapy [55]. In contrast, PEG-IFNa treatment resulted in a larger magnitude of qHBsAg decline. After 48 weeks of PEG-IFNa treatment, a 0.71 log decline in qHBsAg levels was observed [56]. In addition, treatment responders were likely to have more significant decline in qHBsAg levels during the early phase of treatment. In view of this characteristic, qHBsAg profile (baseline level and on-treatment decline) has been incorporated in treatment algorithms to indicate treatment futility and for consideration of treatment cessation. In HBeAg-positive CHB patients, qHBsAg level >20,000 for genotype B/C or no decline of qHBsAg for genotype A/D at 12 weeks of PEG-IFNa fulfils the treatment-stopping criteria. If the week 24 qHBsAg remains >20,000 IU/L, PEG-IFNa should also be stopped regardless of genotype. Similarly, for HBeAg-negative CHB patients with genotype D infection, absence of qHBsAg decline in combination with <2 log reduction in serum HBV DNA at 12 weeks should also be regarded as futile [26].
HBcrAg
Hepatitis B core-related antigen (HBcrAg) is a composite of 3 related proteins that share an identical 149 amino acid sequence: HBcAg, HBeAg and a truncated 22 kDa precore protein (p22Cr) that is a processed product of the precore protein; see Figure 1. The chemiluminescence signal is generated from immunocomplexes formed between HBcrAg and alkaline phosphatase-labelled anti-HBcrAg antibodies, after which the quantity can be derived from known concentrations of recombinant ProHBeAg [57,58]. HBcrAg demonstrates good correlation with intrahepatic cccDNA (r=0.48–0.70) in both untreated and NUC-treated subjects [57].
Measurement of HBcrAg at 48 weeks of first-line NUCs or 52 weeks of PEG-IFNa therapy demonstrated a median decline of 1.37 log [33,59]. Reduction in HBcrAg was correlated with reduction in cccDNA (r=0.503) [60]. The main limitation with HBcrAg is the relatively high lower limit of detection (3 log U/mL), and is not detectable in up to 30% of HBeAg-negative patients [33]. A recent novel HBcrAg assay demonstrated an improved sensitivity of 2.1 log U/mL, and potentially will provide more insights in the viral kinetics and changes upon treatment especially in HBeAg-negative patients [61].
Table 1 summarizes the treatment effects on hepatitis B viral biomarkers at 1 year stratified by treatment type.
POTENTIAL APPLICATION OF VIRAL MARKERS
Blood-based HBV biomarkers are crucial for evaluating treatment candidacy, treatment response in both approved therapies and novel drugs in the pipeline.
Decision on treatment candidacy
Not all CHB subjects are eligible for antiviral treatment. In the various clinical guidelines, serum viral biomarkers are essential to determine treatment candidacy. Serum HBV DNA remains the most important parameter, although qualitative HBeAg is included in the American Association for the Study of Liver Diseases (AASLD) and APASL guidelines to decide on the threshold of HBV DNA above which treatment is indicated. In general, serum HBV DNA >20,000 IU/mL (for HBeAg-positive subjects) or >2,000 IU/mL (for HBeAg-negative subjects) plus elevated serum alanine aminotransferase or presence of other risk features would be considered eligible for treatment. In cirrhotic patients, the HBV DNA threshold for treatment would be much lowered [26,62,63]. Higher serum HBcrAg were independently associated with immune tolerance over immune clearance among HBeAg-positive patients (8.2 vs 7.6 log) [64]. In contrast, lower serum qHBsAg levels were independently associated with inactive carrier state and HBsAg seroclearance [65-68]. These biomarkers might play a role to identify patients requiring antiviral therapy in the HBeAg-positive and HBeAg-negative phase, respectively.
In the setting of prevention of mother-to-child-transmission, the WHO recommends HBV DNA testing to decide whether antiviral prophylaxis should be given during pregnancy [69]. HBV DNA >200,000 IU/mL is regarded the threshold to initiate TDF treatment. Where antenatal HBV DNA testing is unavailable, both HBeAg (qualitative) and qHBsAg can be used as a surrogate marker to determine eligibility of TDF prophylaxis. HBeAg positivity has a sensitivity of 88.2% and specificity of 92.6% to detect HBV DNA >200,000 IU/mL [70]. Likewise, serum qHBsAg >4 log is 85.1% sensitive and 96.5% specific for HBV DNA >200,000 IU/mL [71,72].
A recently developed Xpert® HBV Viral Load assay for HBV DNA has shown promise to accurately quantify HBV DNA in dried blood spots, with 85.4% having estimable viral loads to within 1 log of the corresponding serum load, with a limit of detection of 7.5 IU/mL [73,74]. This approach would be very helpful in many resource-limited settings especially where the GeneXpert® system is already in place for the purpose of analysing other pathogens such as SARS-CoV-2 or Mycobacterium tuberculosis & rifampin resistance. Another point-of-care rapid diagnostic test (RDT) for HBcrAg has recently been developed using stored sera as a simplified assessment tool especially in settings where HBV DNA or qHBsAg are not routinely available. With a detection limit of 4.3 log U/mL, the RDT-HBcrAg can identify highly viremic patients that fulfil treatment criteria according to clinical guidelines, with sensitivity 90.5–96.6% and specificity 83.2–96.8% [75]. The RDT-HBcrAg kit has a low production cost (<USD 5), reasonable operating temperature (18–39°C) with simple sample handling without needing any specific equipment or molecular laboratory facilities. More validation studies for this kit as well as the cost-effectiveness of this approach in resource-limited settings should be evaluated.
Dose adjustment and regimen modification
With the understanding of the on-treatment profile of HBV DNA and qHBsAg, both are essential markers to be monitored during the course of therapy [26]. In NUC recipients, HBV DNA monitoring is essential to detect virological breakthrough which would suggest either primary resistance or non-compliance to treatment. In PEG-IFNa recipients, stopping rules are defined according to qHBsAg levels as discussed above.
Risk stratification for HCC
Viral biomarkers give important clues in the risk of HCC among treated CHB subjects. Serum qHBsAg has been shown to associated with HCC risk. The hazard ratio for developing HCC was 13.7 for low viremic (HBV DNA <2,000 IU/mL) HBeAg-negative patients with serum qHBsAg ≥3 log compared to those with serum qHBsAg <3 log [76]. Moreover, HBsAg seroclearance, i.e., functional cure, is associated with significantly reduced HCC risk, especially in subjects who achieved this endpoint before the age of 50 and regardless of whether the patient was given antiviral therapy [77,78]. Serum viral load (HBV DNA) is a well-known risk factor for HCC and demonstrated a biological gradient in the REVEAL-HBV cohort [79]. Long term NUC treatment has been shown to reduce the risk of HCC [80]. Since HBV DNA is no longer detectable in the serum (in the majority of cases) upon NUC treatment, other viral biomarkers have been explored to assess the risk of HCC in antiviral-treated CHB patients. In this context, serum HBcrAg and pgRNA might aid risk stratification in addition to serum HBV DNA and qHBsAg levels [81,82]. While serum HBcrAg is reduced in all NUC-treated CHB patients [83], a high post-treatment HBcrAg was associated with >2 fold increase in risk of HCC [84]. Similarly, on-treatment detectable serum pgRNA is associated with 3.5-fold higher risk of HCC in 2 years’ time [85].
Prediction of partial/functional cure
Among HBeAg-positive patients, a higher baseline serum HBcrAg was independently associated with NA-induced HBeAg seroconversion [64], while a lower HBcrAg at week 12 of PEG-IFN was predictive of HBeAg seroclearance and HBV DNA <2,000 IU/mL at 24 weeks post-treatment [60]. HBeAg seroclearance/seroconversion is the pre-requisite for cessation of long-term NUC among HBeAg-positive patients, after HBV DNA undetectability for a certain period, in order to achieve incomplete cure. Numerous studies have explored the success rate and predictors for off-therapy virological control [14,86]. Apart from host factors, viral factors might provide insights in risk of virological or clinical relapse after stopping long term NUC. Low end-of-therapy (EOT) serum qHBsAg, preferably <100 IU/mL, has been consistently shown to predict partial cure [87-89]. In addition, low EOT serum HBcrAg [90], undetectable EOT serum HBV pgRNA [91], or a combination of both [92,93], identified a subgroup of patients who would be able to stop long-term NUC with a lower chance of flare. Some patients with a favourable viral biomarker profile would benefit from such approach and achieve functional cure [94,95]. In fact, assessing viral biomarkers (serum HBcrAg and pgRNA) as early as week 4 of NUC treatment is able to highlight a group of patients who would achieve a low serum qHBsAg (<100 IU/mL) or HBsAg seroclearance in the long run [96]. This approach can help to identify subjects during the early phase who should not stop NUC and should be prioritized into clinical trials.
Evaluation of efficacy and target engagement for novel compounds
The treatment landscape of CHB is expected to change with the numerous novel agents being explored; detailed discussion of these therapeutic approaches has been reviewed elsewhere [97,98]. These drugs target alternative steps in the viral replication cycle, stimulate host immune response, or act on both pathways. As mentioned above, functional cure is the desirable treatment endpoint for phase 3 clinical trials of novel CHB therapy [18].
At the time of writing, several novel compounds have demonstrated promising results on sustainable HBsAg suppression. RNA interference-based therapy with either small interfering RNAs or antisense oligonucleotide (ASO) were able to knock down HBsAg levels by more than 1 log within <48 weeks of treatment [98]. For instance, JNJ-3989, a siRNA, when given with NUC led to HBsAg reduction by ≥1 log from baseline in 39/40 (97.5%) subjects at nadir, which persisted in 38% patients at 1 year post EOT [99]. The mean declines of HBeAg, HBcrAg and HBV RNA from baseline to 16 weeks were 1.47 log PEIU/mL, 1.2 log kU/mL and 1.93 log U/mL respectively. Bepirovirsen, an ASO, was able to induce functional cure in 9–10% participants assessed at 24 weeks post-EOT [100].
However, despite the large number of ongoing trials, no compounds have reached the benchmark of inducing functional cure in ≥30% subjects. It is therefore important to understand the mechanisms of action for various novel compounds and utilize the appropriate viral biomarkers to evaluate target engagement as an interim response [101]. Core protein allosteric modulator (CpAM) inhibits the formation of functional capsids and encapsidation, thereby reducing the amount of circulating encapsidated pgRNA. In patients who received vebicorvir (CpAM), significant reductions in serum HBV DNA and pgRNA were observed at week 12 and 24 even though no change in serum qHBsAg was seen [102]. The inhibition of pgRNA synthesis could be observed as early as day 15 in patients receiving ABI-H2158 (CpAM), with mean decline of >2 log from baseline compared to 0.03 log in the placebo group [103]. Other viral markers such as HBeAg and HBcrAg levels were evaluated in some of the trials involving CpAM [104,105].
According to a recent study with treatment-naïve cohorts receiving 4 8 week s of NUC s+RO70 49389 (CpAM) or NUCs+RO7049389+PEG-IFNa, the mean declines of HBeAg were 1.48 and 2.10 log IU/mL and for HBcrAg 1.23 and 1.76 log U/mL respectively [106]. However, the long-term durability of the viral kinetic changes during novel therapies, as well as predictive factors for a durable suppression of various viral biomarkers, remains largely unclear and should be evaluated in future clinical trials.
Table 2 summarizes the potential applications of the viral biomarkers discussed in various settings.
CONCLUSION
Viral biomarker assessment is indispensable in clinical management and through the journey of novel drug discovery in the field of CHB. In the current era with highly effective NUC therapy as the mainstay of treatment, HBV DNA will be expectedly undetectable and novel transcriptional (HBV RNA) and translational markers (qHBsAg and HBcrAg) can provide further insights into treatment efficacy. Emerging data suggests these viral biomarkers can aid treatment decision, risk stratification for HCC and risk prediction for partial cure/functional cure. As the primary outcome of phase III trials is set on functional cure, viral biomarkers can potentially inform the efficacy of novel compounds or treatment approaches in the early course of treatment, and help with prioritization of subjects into clinical trials. Importantly, standardization and validation studies are necessary before viral biomarkers can be broadly implemented in clinical use. The role of viral biomarkers needs to be further explored to pave the way into elimination of viral hepatitis B.
Notes
Authors’ contribution
LYM was responsible literature search, critical appraisal and drafting of the manuscript. RWHH, JF and WKS were responsible for critical revision of the article. MFY was responsible for conception of the work and critical approval of the article.
Conflicts of Interest
LY Mak is an advisory board member for Gilead Sciences. WK Seto received speaker’s fees from AstraZeneca and Mylan, is an advisory board member of CSL Behring, is an advisory board member and received speaker’s fees from AbbVie, and is an advisory board member, received speaker’s fees and researching funding from Gilead Sciences. MF Yuen serves as advisor/consultant for AbbVie, Assembly Biosciences, Aligos Therapeutics, Arbutus Biopharma, Bristol Myer Squibb, Clear B Therapeutics, Dicerna Pharmaceuticals, Finch Therapeutics, GlaxoSmithKline, Gilead Sciences, Immunocore, Janssen, Merck Sharp and Dohme, Hoffmann-La Roche and Springbank Pharmaceuticals, Vir Biotechnology and receives grant/research support from Assembly Biosciences, Aligos Therapeutics, Arrowhead Pharmaceuticals, Bristol Myer Squibb, Fujirebio Incorporation, Gilead Sciences, Immunocore, Merck Sharp and Dohme, Hoffmann-La Roche, Springbank Pharmaceuticals and Sysmex Corporation. The remaining authors have no conflict of interests.
Abbreviations
CHB
chronic hepatitis B
HCC
hepatocellular carcinoma
WHO
World Health Organization
HBV
hepatitis B virus
DNA
double-stranded deoxy-ribonucleic acid
rcDNA
relaxed circular DNA
NTCP
sodium taurocholate co-transporting polypeptide
cccDNA
covalently closed circular DNA
pgRNA
pre-genomic RNA
HBsAg
hepatitis B surface antigen
HBeAg
hepatitis B e antigen
HBcAg
hepatitis core antigen
HBx
x protein
dslDNA
double-stranded linear DNA
NUCs
nucleos(t) ide analogues
PEG-IFNa
pegylated interferon alpha
ETV
entecavir
TDF
tenofovir disoproxil fumarate
TAF
tenofovir alafenamide
RACE
rapid amplification of complimentary DNA ends
SVP
subviral particles
qHBsAg
quantitative hepatitis B surface antigen
HBcrAg
hepatitis B core related antigen
P22Cr
precore protein
RDT
rapid diagnostic test
EOT
end-of-therapy
RNAi
RNA interference
siRNAs
small interfering RNAs
ASO
antisense oligonucleotide
CpAM
core protein allosteric modulator