1. Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol 2018;3:383-403.

2. Global Hepatitis Report 2017. Geneva: World Health Organization; 2017.
3. Razavi-Shearer D, Gamkrelidze I, Blach S, Estes C, Mooneyhan E, Razavi-Shearer K, et al. The incidence of chronic HBV by age at the global and regional level, 2022. Hepatology Vol. 76. Wiley. 2022:S29.
4. Ni Y, Lempp FA, Mehrle S, Nkongolo S, Kaufman C, F├żlth M, et al. Hepatitis B and D viruses exploit sodium taurocholate cotransporting polypeptide for species-specific entry into hepatocytes. Gastroenterology 2014;146:1070-1083.


7. K├Čniger C, Wingert I, Marsmann M, R├Čsler C, Beck J, Nassal M. Involvement of the host DNA-repair enzyme TDP2 in formation of the covalently closed circular DNA persistence reservoir of hepatitis B viruses. Proc Natl Acad Sci U S A 2014;111:E4244-4253.


8. Seeger C, Mason WS. Molecular biology of hepatitis B virus infection. Virology 2015;479-480:672-686.


9. Tuttleman JS, Pourcel C, Summers J. Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells. Cell 1986;47:451-460.


13. Schmid J, Langhorst J, Ga├¤ F, Theysohn N, Benson S, Engler H, et al. Placebo analgesia in patients with functional and organic abdominal pain: a fMRI study in IBS, UC and healthy volunteers. Gut 2015;64:418-427.


14. Berg T, Simon KG, Mauss S, Schott E, Heyne R, Klass DM, et al.; FINITE CHB study investigators. Long-term response after stopping tenofovir disoproxil fumarate in non-cirrhotic HBeAg-negative patients - FINITE study. J Hepatol 2017;67:918-924.


15. Bonvin M, Achermann F, Greeve I, Stroka D, Keogh A, Inderbitzin D, et al. Interferon-inducible expression of APOBEC3 editing enzymes in human hepatocytes and inhibition of hepatitis B virus replication. Hepatology 2006;43:1364-1374.


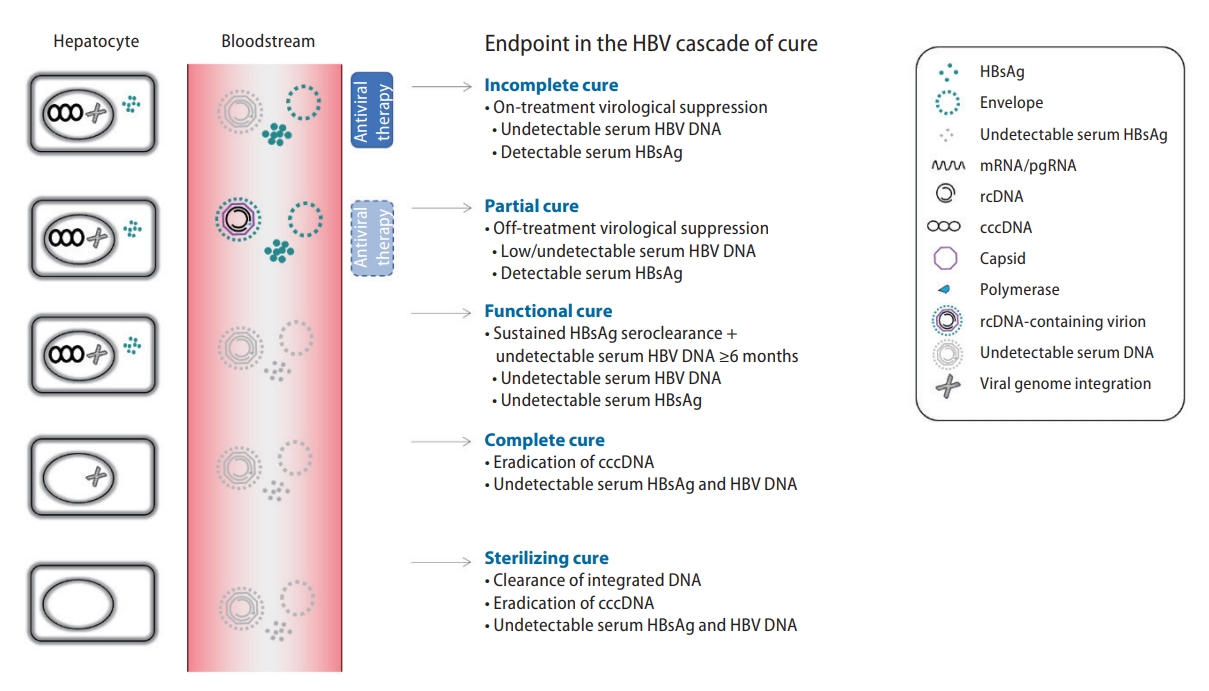
18. Cornberg M, Lok AS, Terrault NA, Zoulim F; 2019 EASL-AASLD HBV Treatment Endpoints Conference Faculty. Guidance for design and endpoints of clinical trials in chronic hepatitis B -Report from the 2019 EASL-AASLD HBV Treatment Endpoints Conference. Hepatology 2019 Nov 12;doi: 10.1002/hep.31030.


19. Neuberger J, Patel J, Caldwell H, Davies S, Hebditch V, Hollywood C, et al. Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology. Gut 2020;69:1382-1403.


20. Mani H, Kleiner DE. Liver biopsy findings in chronic hepatitis B. Hepatology 2009;49(5 Suppl):S61-71.


21. Zhao XL, Yang JR, Lin SZ, Ma H, Guo F, Yang RF, et al. Serum viral duplex-linear DNA proportion increases with the progression of liver disease in patients infected with HBV. Gut 2016;65:502-511.


22. Wong DK, Yuen MF, Yuan H, Sum SS, Hui CK, Hall J, et al. Quantitation of covalently closed circular hepatitis B virus DNA in chronic hepatitis B patients. Hepatology 2004;40:727-737.


23. Lin LY, Wong VW, Zhou HJ, Chan HY, Gui HL, Guo SM, et al. Relationship between serum hepatitis B virus DNA and surface antigen with covalently closed circular DNA in HBeAg-negative patients. J Med Virol 2010;82:1494-1500.


24. Guner R, Karahocagil M, Buyukberber M, Kandemir O, Ural O, Usluer G, et al. Correlation between intrahepatic hepatitis B virus cccDNA levels and other activity markers in patients with HBeAg-negative chronic hepatitis B infection. Eur J Gastroenterol Hepatol 2011;23:1185-1191.


26. European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370-398.


27. Wang J, Shen T, Huang X, Kumar GR, Chen X, Zeng Z, et al. Serum hepatitis B virus RNA is encapsidated pregenome RNA that may be associated with persistence of viral infection and rebound. J Hepatol 2016;65:700-710.


28. Giersch K, Allweiss L, Volz T, Dandri M, L├╝tgehetmann M. Serum HBV pgRNA as a clinical marker for cccDNA activity. J Hepatol 2017;66:460-462.


31. van B├Čmmel F, Bartens A, Mysickova A, Hofmann J, Kr├╝ger DH, Berg T, et al. Serum hepatitis B virus RNA levels as an early predictor of hepatitis B envelope antigen seroconversion during treatment with polymerase inhibitors. Hepatology 2015;61:66-76. Erratum in: Hepatology 2016;63:349.
33. Mak LY, Cloherty G, Wong DK, Gersch J, Seto WK, Fung J, et al. HBV RNA profiles in patients with chronic hepatitis B under different disease phases and antiviral therapy. Hepatology 2021;73:2167-2179. Erratum in: Hepatology 2021;74:3561.
36. Ma H, Yang RF, Wei L. Quantitative serum HBsAg and HBeAg are strong predictors of sustained HBeAg seroconversion to pegylated interferon alfa-2b in HBeAg-positive patients. J Gastroenterol Hepatol 2010;25:1498-1506.


37. Geng M, Li Y, Gao F, Sun L, Yang X, Wang R, et al. A scoring model predicts hepatitis B e antigen seroconversion in chronic hepatitis B patients treated with nucleos(t)ide analogs: real-world clinical practice. Int J Infect Dis 2017;62:18-25.


40. Roche. Elecsys® HBsAg II: Immunoassay for the qualitative determination of hepatitis B surface antigen (HBsAg).
42. Wursthorn K, Jaroszewicz J, Zacher BJ, Darnedde M, Raupach R, Mederacke I, et al. Correlation between the Elecsys HBsAg II assay and the Architect assay for the quantification of hepatitis B surface antigen (HBsAg) in the serum. J Clin Virol 2011;50:292-296.


43. Lou S, Taylor R, Pearce S, Kuhns M, Leary T. An ultra-sensitive Abbott ARCHITECT
® assay for the detection of hepatitis B virus surface antigen (HBsAg). J Clin Virol 2018;105:18-25.


47. Thompson AJ, Nguyen T, Iser D, Ayres A, Jackson K, Littlejohn M, et al. Serum hepatitis B surface antigen and hepatitis B e antigen titers: disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology 2010;51:1933-1944.


48. Chan HL, Fung S, Seto WK, Chuang WL, Chen CY, Kim HJ, et al.; GS-US-320-0110 Investigators. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol 2016;1:185-195. Erratum in: Lancet Gastroenterol Hepatol 2016;1:e2.
53. Buti M, Gane E, Seto WK, Chan HL, Chuang WL, Stepanova T, et al.; GS-US-320-0108 Investigators. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol 2016;1:196-206. Erratum in: Lancet Gastroenterol Hepatol 2016;1:e2.
54. Wong VW, Wong GL, Yan KK, Chim AM, Chan HY, Tse CH, et al. Durability of peginterferon alfa-2b treatment at 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology 2010;51:1945-1953.


56. Brunetto MR, Moriconi F, Bonino F, Lau GK, Farci P, Yurdaydin C, et al. Hepatitis B virus surface antigen levels: a guide to sustained response to peginterferon alfa-2a in HBeAg-negative chronic hepatitis B. Hepatology 2009;49:1141-1150.


61. Inoue T, Kusumoto S, Iio E, Ogawa S, Suzuki T, Yagi S, et al. Clinical efficacy of a novel, high-sensitivity HBcrAg assay in the management of chronic hepatitis B and HBV reactivation. J Hepatol 2021;75:302-310.


62. Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560-1599.


63. Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1-98.


66. Chan HL, Thompson A, Martinot-Peignoux M, Piratvisuth T, Cornberg M, Brunetto MR, et al. Hepatitis B surface antigen quantification: why and how to use it in 2011 - a core group report. J Hepatol 2011;55:1121-1131.


69. Prevention of mother-to-child transmission of hepatitis B virus: guidelines on antiviral prophylaxis in pregnancy. Geneva: World Health Organization; 2020.
70. Boucheron P, Lu Y, Yoshida K, Zhao T, Funk AL, Lunel-Fabiani F, et al. Accuracy of HBeAg to identify pregnant women at risk of transmitting hepatitis B virus to their neonates: a systematic review and meta-analysis. Lancet Infect Dis 2021;21:85-96.


71. Sun KX, Li J, Zhu FC, Liu JX, Li RC, Zhai XJ, et al. A predictive value of quantitative HBsAg for serum HBV DNA level among HBeAg-positive pregnant women. Vaccine 2012;30:5335-5340.


73. Jackson K, Tekoaua R, Li X, Locarnini S. Real-world application of the Xpert
® HBV viral load assay on serum and dried blood spots. J Med Virol 2021;93:3707-3713.



74. Abravanel F, Lhomme S, Tr├®meaux P, Migueres M, Harter A, Hasl├® C, et al. Performance of the Xpert HBV Viral Load assay versus the Aptima Quant assay for quantifying hepatitis B virus DNA. Diagn Microbiol Infect Dis 2020;96:114946.


75. Shimakawa Y, Ndow G, Kaneko A, Aoyagi K, Lemoine M, Tanaka Y; the PROLIFICA/HBcrAg-RDT Study Group. Rapid pointof-care test for hepatitis B core-related antigen to diagnose high viral load in resource-limited settings. Clin Gastroenterol Hepatol 2022 Jun 11;doi: 10.1016/j.cgh.2022.05.026.

76. Tseng TC, Liu CJ, Yang HC, Su TH, Wang CC, Chen CL, et al. High levels of hepatitis B surface antigen increase risk of hepatocellular carcinoma in patients with low HBV load. Gastroenterology 2012;142:1140-1149.e3 quiz e13-4.


77. Yuen MF, Wong DK, Fung J, Ip P, But D, Hung I, et al. HBsAg Seroclearance in chronic hepatitis B in Asian patients: replicative level and risk of hepatocellular carcinoma. Gastroenterology 2008;135:1192-1199.


78. Yip TC, Chan HL, Wong VW, Tse YK, Lam KL, Wong GL. Impact of age and gender on risk of hepatocellular carcinoma after hepatitis B surface antigen seroclearance. J Hepatol 2017;67:902-908.


79. Chen CJ, Yang HI, Iloeje UH; REVEAL-HBV Study Group. Hepatitis B virus DNA levels and outcomes in chronic hepatitis B. Hepatology 2009;49(5 Suppl):S72-84.


80. Su TH, Kao JH. Improving clinical outcomes of chronic hepatitis B virus infection. Expert Rev Gastroenterol Hepatol 2015;9:141-154.


86. Liem KS, Fung S, Wong DK, Yim C, Noureldin S, Chen J, et al. Limited sustained response after stopping nucleos(t)ide analogues in patients with chronic hepatitis B: results from a randomised controlled trial (Toronto STOP study). Gut 2019;68:2206-2213.


87. Chen CH, Hung CH, Hu TH, Wang JH, Lu SN, Su PF, et al. Association between level of hepatitis B surface antigen and relapse after entecavir therapy for chronic hepatitis B virus infection. Clin Gastroenterol Hepatol 2015;13:1984-1992.e1.


88. Chen CH, Hung CH, Wang JH, Lu SN, Hu TH, Lee CM. Long-term incidence and predictors of hepatitis B surface antigen loss after discontinuing nucleoside analogues in noncirrhotic chronic hepatitis B patients. Clin Microbiol Infect 2018;24:997-1003.


89. Jeng WJ, Chen YC, Sheen IS, Lin CL, Hu TH, Chien RN, et al. Clinical relapse after cessation of tenofovir therapy in hepatitis B e antigen-negative patients. Clin Gastroenterol Hepatol 2016;14:1813-1820.e1.


90. Sonneveld MJ, Park JY, Kaewdech A, Seto WK, Tanaka Y, Carey I, et al.; CREATE Study Group. Prediction of sustained response after nucleo(s)tide analogue cessation using HBsAg and HBcrAg levels: a multicenter study (CREATE). Clin Gastroenterol Hepatol 2022;20:e784-e793.


91. Seto WK, Liu KS, Mak LY, Cloherty G, Wong DK, Gersch J, et al. Role of serum HBV RNA and hepatitis B surface antigen levels in identifying Asian patients with chronic hepatitis B suitable for entecavir cessation. Gut 2021;70:775-783.


93. Papatheodoridi M, Papachristou E, Moschidis Z, Hadziyannis E, Rigopoulou E, Zachou K, et al. Significance of serum HBV RNA in non-cirrhotic HBeAg-negative chronic hepatitis B patients who discontinue effective antiviral therapy. J Viral Hepat 2022;29:948-957.

94. Hirode G, Choi HSJ, Chen CH, Su TH, Seto WK, Van Hees S, et al.; RETRACT-B Study Group. Off-therapy response after nucleos(t) ide analogue withdrawal in patients with chronic hepatitis B: an international, multicenter, multiethnic cohort (RETRACT-B Study). Gastroenterology 2022;162:757-771.e4.


95. Berg T, Lampertico P. The times they are a-changing - A refined proposal for finite HBV nucleos(t)ide analogue therapy. J Hepatol 2021;75:474-480.


98. Mak LY, Cheung KS, Fung J, Seto WK, Yuen MF. New strategies for the treatment of chronic hepatitis B. Trends Mol Med 2022;28:742-757.


99. Yuen MF, Locarnini S, Lim TH, Strasser SI, Sievert W, Cheng W, et al. Combination treatments including the small-interfering RNA JNJ-3989 induce rapid and sometimes prolonged viral responses in patients with CHB. J Hepatol 2022;77:1287-1298.


100. Yuen MF, Lim SG, Plesniak R, Tsuji K, Janssen HLA, Pojoga C, et al.; B-Clear Study Group. Efficacy and safety of bepirovirsen in chronic hepatitis B infection. N Engl J Med 2022;387:1957-1968.


102. Sulkowski MS, Agarwal K, Ma X, Nguyen TT, Schiff ER, Hann HL, et al. Safety and efficacy of vebicorvir administered with entecavir in treatment-naïve patients with chronic hepatitis B virus infection. J Hepatol 2022;77:1265-1275.


103. Agarwal K, Xu J, Gane EJ, Nguyen TT, Ding Y, Knox SJ, et al. Safety, pharmacokinetics and antiviral activity of ABI-H2158, a hepatitis B virus core inhibitor: A randomized, placebocontrolled phase 1 study. J Viral Hepat 2023;30:209-222.

104. Zoulim F, Lenz O, Vandenbossche JJ, Talloen W, Verbinnen T, Moscalu I, et al. JNJ-56136379, an HBV capsid assembly modulator, is well-tolerated and has antiviral activity in a phase 1 study of patients with chronic infection. Gastroenterology 2020;159:521-533.e9.


105. Yuen MF, Gane EJ, Kim DJ, Weilert F, Yuen Chan HL, Lalezari J, et al. Antiviral activity, safety, and pharmacokinetics of capsid assembly modulator NVR 3-778 in patients with chronic HBV infection. Gastroenterology 2019;156:1392-1403.e7.


106. Hou J, Gane EJ, Zhang W, Zhang J, Yuen MF, Lim TH, et al. Hepatitis B virus antigen reduction effect of RO7049389 plus NUC with/without Peg-IFN in chronic hepatitis B patients. J Hepatol 2022;77:S299.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



