| Clin Mol Hepatol > Volume 29(3); 2023 > Article |
|
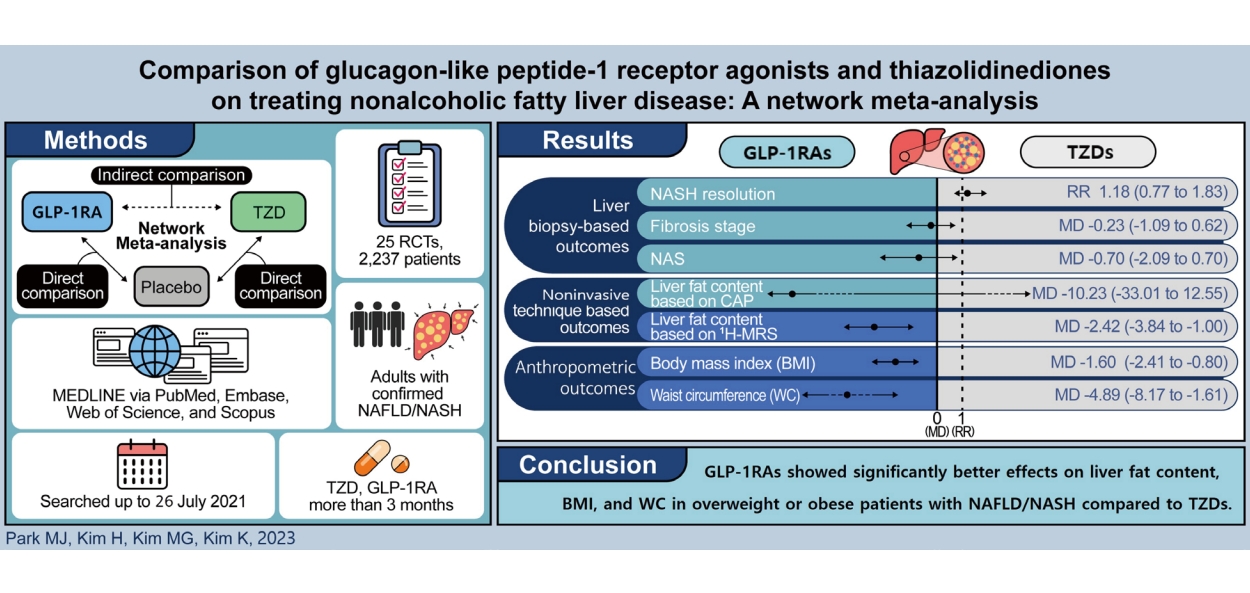
ABSTRACT
Background/Aims
Methods
Results
ACKNOWLEDGMENTS
FOOTNOTES
SUPPLEMENTAL MATERIAL
Supplementary┬ĀMaterial┬Ā2.
Supplementary┬ĀMaterial┬Ā3.
Supplementary┬ĀMaterial┬Ā4.
Supplementary┬ĀMaterial┬Ā5.
Supplementary┬ĀMaterial┬Ā6.
Supplementary┬ĀMaterial┬Ā7.
Supplementary┬ĀMaterial┬Ā8.
Supplementary┬ĀMaterial┬Ā9.
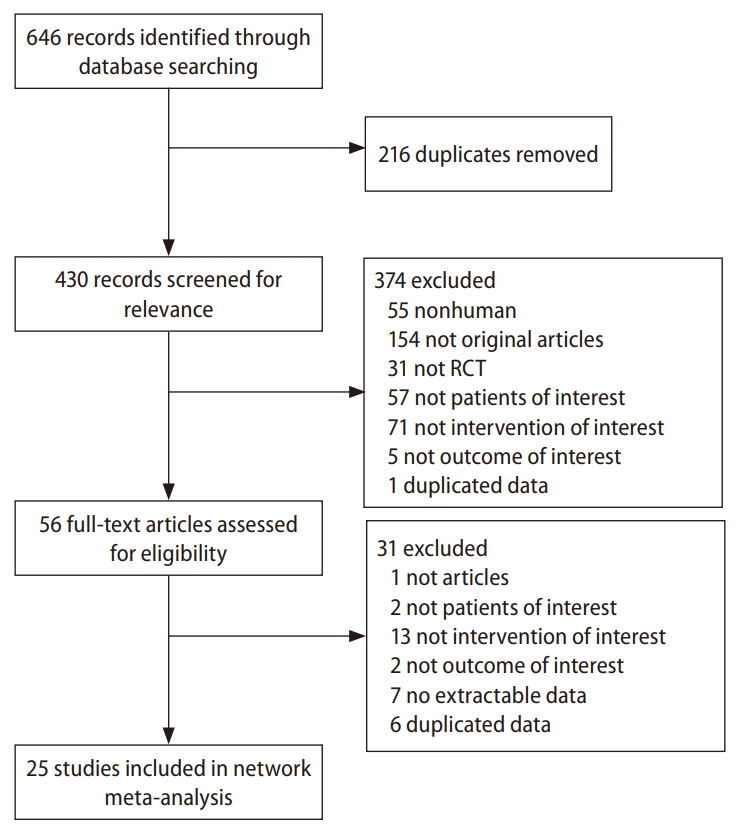
Figure┬Ā2.
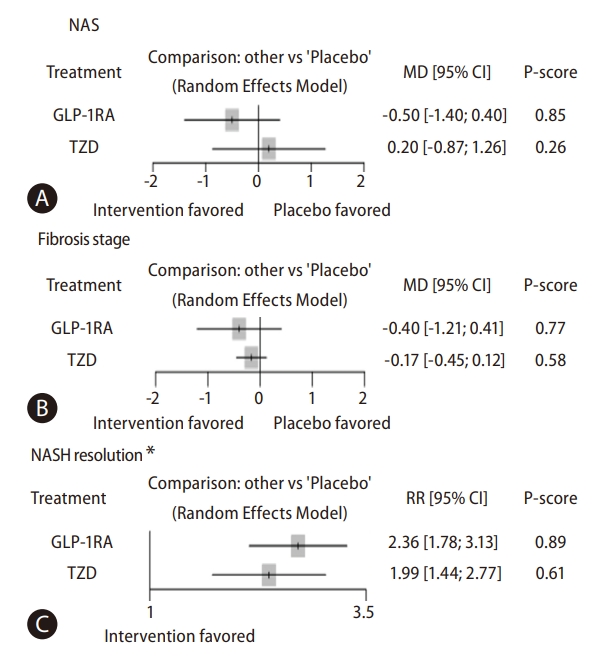
Figure┬Ā3.
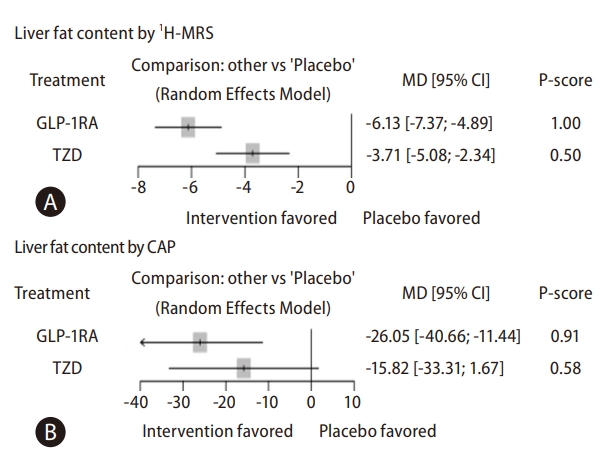
Table┬Ā1.
PICOS, population, intervention, comparison, outcome, and study design; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; TZD, thiazolidinedione; GLP-1RA, glucagon-like peptide-1 receptor agonist; NAS, nonalcoholic fatty liver activity score; 1H-MRS, proton magnetic resonance spectroscopy; CAP, controlled attenuation parameter; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HbA1c, hemoglobin A1c; FPG, fasting plasma glucose; HOMA-IR, homeostasis model assessment of insulin resistance; T-Chol, total cholesterol; LDL-C, low-density lipoprotein-cholesterol; TG, triglyceride; BMI, body mass index; WC, waist circumference.
Table┬Ā2.
| Comparison | k | p | q | NMA MD (95% CI) | Direct MD (95% CI) | Indirect MD (95% CI) | |
|---|---|---|---|---|---|---|---|
| NAS | 4 | NA | |||||
| TZDs vs. placebo | 3 | 1.00 | 0.20 (-0.87 to 1.26) | 0.20 (-0.87 to 1.26) | NA | ||
| GLP-1RAs vs. placebo | 1 | 1.00 | -0.50 (-1.40 to 0.40) | -0.50 (-1.40 to 0.40) | NA | ||
| GLP-1RAs vs. TZDs | - | -0.70 (-2.09 to 0.70) | NA | -0.70 (-2.09 to 0.70) | |||
| Fibrosis stage | 9 | NA | |||||
| TZDs vs. placebo | 8 | 1.00 | -0.17 (-0.45 to 0.12) | -0.17 (-0.45 to 0.12) | NA | ||
| GLP-1RAs vs. placebo | 1 | 1.00 | -0.40 (-1.21 to 0.41) | -0.40 (-1.21 to 0.41) | NA | ||
| GLP-1RAs vs. TZDs | - | -0.23 (-1.09 to 0.62) | NA | -0.23 (-1.09 to 0.62) | |||
| NASH resolution | 7 | NA | |||||
| TZDs vs. placebo | 3 | 1.00 | 1.99 (1.44 to 2.77)* | 1.99 (1.44 to 2.77)* | NA | ||
| GLP-1RAs vs. placebo | 4 | 1.00 | 2.36 (1.78 to 3.13)* | 2.36 (1.78 to 3.13)* | NA | ||
| GLP-1RAs vs. TZDs | - | 1.18 (0.77 to 1.83) | NA | 1.18 (0.77 to 1.83) | |||
| Liver fat content based on 1H-MRS | 5 | 0.04 | |||||
| TZDs vs. placebo | 3 | 0.88 | -3.71 (-5.08 to -2.34)* | -3.61 (-5.30 to -1.92)* | -3.90 (-6.24 to -1.56)* | ||
| GLP-1RAs vs. placebo | 1 | 0.53 | -6.13 (-7.37 to -4.89)* | -6.20 (-7.63 to -4.77)* | -5.91 (-8.41 to -3.40)* | ||
| GLP-1RAs vs. TZDs | 1 | 0.59 | -2.42 (-3.84 to -1.00)* | -2.30 (-4.15 to -0.45)* | -2.59 (-4.81 to -0.38)* | ||
| Liver fat contents based on CAP | 3 | NA | |||||
| TZDs vs. placebo | 1 | 1.00 | -15.82 (-33.31 to 1.67) | -15.82 (-33.31 to 1.67) | NA | ||
| GLP-1RAs vs. placebo | 2 | 1.00 | -26.05 (-40.66 to -11.44)* | -26.05 (-40.66 to -11.44)* | NA | ||
| GLP-1RAs vs. TZDs | - | -10.23 (-33.01 to 12.55) | NA | -10.23 (-33.01 to 12.55) | |||
MD, mean difference; CI, confidence interval; NAS, nonalcoholic fatty liver activity score; TZD, thiazolidinedione; GLP-1RA, glucagon-like peptide-1 receptor agonist; NA, not addressed; NASH, nonalcoholic steatohepatitis; 1H-MRS, proton magnetic resonance spectroscopy; CAP, controlled attenuation parameter.
k: number of direct comparison studies, p: proportion of direct evidence, q: the CochranŌĆÖs Q statistics between designs, NMA: treatment effects estimated from network meta-analysis, direct: treatment effects estimated from direct comparison, indirect: treatment effects estimated from indirect comparisons.
Data are MD with 95% CI, except for NASH resolution, which reports data in relative risk with 95% CI.
Abbreviations
REFERENCES
- TOOLS
-
METRICS

- ORCID iDs
-
Kyungim Kim

https://orcid.org/0000-0001-7997-696X - Related articles
-
Implications of comorbidities in nonalcoholic fatty liver disease2023 April;29(2)
Global incidence and prevalence of nonalcoholic fatty liver disease2023 February;29(Suppl)
Comparison between obese and non-obese nonalcoholic fatty liver disease2023 February;29(Suppl)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement1
Supplement1 Print
Print



