The imitator of immune-tolerant chronic hepatitis B: A killer in disguise
Article information
Chronic hepatitis B (CHB) is the leading cause of hepatocellular carcinoma (HCC), especially in the Asia-Pacific region [1]. For CHB patients in the clinical immune-tolerant (IT) phase or HBeAg-positive chronic hepatitis B virus (HBV) infection phase, the Asian Pacific Association for the Study of the Liver and the Korean Association for the Study of the Liver guidelines recommend considering liver biopsies to determine antiviral treatment if there are risk factors [2,3]. The guidelines from the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL) suggest that antiviral treatment may be initiated in selected patients based on their age and/or the histological degree of hepatic fibrosis and inflammation [4,5]. Despite a number of debates in terms of the prevention for liver-related events, there may be general agreement that antiviral therapy is unlikely to be beneficial for patients in the “true” IT phase of CHB, which can be identified by the absence of both active hepatic inflammation (which reflects current hepatic injury) and significant hepatic fibrosis (which reflects previous liver injury). Therefore, it is necessary to define the true IT phase succinctly in everyday practice to optimize the management of CHB patients.
In clinical practice, however, it may be challenging to clearly distinguish between the “true” IT phase and the “fake” IT phase (or indolent immune-active phase) because blood biochemical assays and imaging examinations are not sensitive enough to capture active inflammation and significant hepatic fibrosis, respectively, without histological evaluation. Several lines of evidence suggest that the clinical IT phase (HBeAg-positive, high HBV DNA level, and normal ALT) may not necessarily represent the true IT phase. In a study involving Asian-American CHB patients in the clinical IT phase, approximately one-fourth of the patients exhibited histologically significant fibrosis (F2 or F3) [6]. A study conducted in Hong Kong showed that 10% of CHB patients with normal ALT had advanced fibrosis on transient elastography [7]. Moreover, surprisingly, in the article that accompanies this editorial, Yoo et al. [8] reported that 177 out of 259 (68.3%) patients who were in the clinical IT phase (defined by HBeAg-positive, HBV DNA level of ≥6 log10 IU/mL, and ALT level of ≤60 U/L) were not in the true histological IT phase (defined by both hepatic fibrosis and inflammation grades of 0 or 1): 174 patients had significant fibrosis ranging from F2 to F4, and 94 patients had moderate or severe inflammation [8].
However, an unexpectedly higher proportion of the fake IT phase in the current study, compared to prior studies, needs to be interpreted with caution. Instead of performing liver biopsy on all consecutive patients in the clinical IT phase, it was left to the discretion of attending physicians, which might be influenced by several hepatic fibrosis-related indicators (e.g., platelet counts and serum albumin level). Under this condition, the proportion of patients belonging to the fake IT phase may be overestimated. In fact, patients in the fake IT phase had lower platelet counts and serum albumin levels than those in the true IT phase. Based on the clinical outcomes of patients in the true vs. fake IT phase, the authors suggested that age over 35, high aspartate aminotransferase (AST), and low albumin levels could be useful in ruling out the histological IT phase.
Specifically, age was identified as a risk factor for liver-related events independent of histological fibrosis grade, supporting the recommendations of AASLD/EASL guidelines, which suggest the initiation of antiviral treatment among CHB patients in the clinical IT phase if the age exceeds 30–40 years. Even though the majority of the Korean CHB patients are infected with genotype C HBV [9] and have a longer IT phase than patients from other regions, the results of this study suggest that the AASLD/EASL recommendation can be applied to Korean CHB patients as well.
In a Korean study, patients with IT phase CHB were reported to have a higher risk of HCC and death compared to those with immune-active phase CHB who were treated with antiviral agents [10]. The IT group had 2.54- and 3.38-fold higher risks of HCC and death or transplantation, respectively, than treated immune-active phase patients. On the other hand, a recent meta-analysis showed comparable risks of HCC and mortality between the untreated IT and the treated immuneactive phases among HBeAg-positive CHB patients without cirrhosis [11]. Another retrospective study reported a negligible incidence of HCC among CHB patients in the IT phase if their fibrosis-4 index is low [12]. This discrepancy may result from the inadequacy of current clinical criteria for the IT phase.
Another Korean study, including CHB patients with ALT below twice the upper limit of normal, demonstrated that the risk of HCC was highest with HBV DNA levels of 6–7 log10 IU/mL and lowest with >8 log10 IU/mL [13], in contrast to the findings of the REVEAL cohort, which showed that HCC risk increased proportionally with HBV DNA level [14]. This difference may stem from the fact that the REVEAL cohort included more HBeAg-negative patients and HBV DNA level >6 log10 IU/mL was not further stratified. Theoretically, prolonged HBV infection may lead to the expansion of HBV-resistant hepatocyte clones, which can reduce HBV replication but promote HCC development [15]. Consequently, HBeAg-positive patients with relatively low levels of HBV DNA (6–7 log10 IU/mL) may experience poor clinical outcomes, and the presence of the fake IT phase should be suspected in these patients. In the accompanying study by Yoo et al. [8], HBV DNA level was not identified as a factor to rule out histological IT phase. This may be due to the relatively low proportion of patients (12.7%) with HBV DNA levels of 6–7 log10 IU/mL. Nonetheless, it is noteworthy that HBV DNA levels of 6–7 log10 IU/mL increased the odds of both being in the fake IT phase (odds ratio=1.96, P=0.3) and having advanced fibrosis (odds ratio=1.93, P=0.068), although the results failed to reach statistical significance.
The results of current and previous studies collectively indicate that at least 10–30% of patients classified as the clinical IT phase may not be in the true IT phase. Clinical diagnosis of the IT phase may be inaccurate, and patients in the fake IT phase can be potential candidates for antiviral treatment. However, several factors should be considered before the initiation of antiviral therapy in CHB patients with high HBV DNA and normal ALT levels. First, under suboptimal activation of the host immune system, antiviral therapy can lead to low viral responses. In a prospective study involving HBeAgpositive patients with normal levels of ALT and high levels of HBV DNA, tenofovir disoproxil fumarate (TDF) with and without emtricitabine showed 76% and 55% of complete virologic response rates, respectively, at week 192 [16]. Fortunately, however, a Korean multicenter retrospective study revealed that the majority of patients achieved complete virologic response after 7–8 years of antiviral treatment [17]. Although several patients infected by TDF-resistant HBV have been confirmed in Korea, all patients had a preexisting multidrugresistance mutation, and none were initially treated with antivirals with a high genetic barrier [18]. Second, long-term side effects of antivirals, such as distinct renal and bone toxicity during TDF treatment, should be considered. Although tenofovir alafenamide has been introduced and shown to be safe for the kidney and bone, it is suspected to be related to weight gain and dyslipidemia [19]. Third, a well-designed prospective study should confirm whether antiviral treatment can reduce the risk of liver-related outcomes among CHB patients in the fake IT phase. Currently, a multinational randomized-controlled trial (ClinicalTrials.gov identif ier: NCT03753074), which investigates the effect of tenofovir alafenamide treatment on the incidence of liver-related events, including HCC, in CHB patients with intermediate-to-high levels of HBV DNA (4–8 log10 IU/mL), normal ALT, and an age of ≥40 years, is ongoing, and the results are awaited.
A recent Korean nationwide cohort study demonstrated that untreated CHB patients had a higher risk of extrahepatic malignancies, which was normalized with antiviral treatment [20]. Future antiviral therapy should be determined based on the expected results of both liver-related (liver cirrhosis, HCC, liver-related death, and liver transplantation) and nonliver-related outcomes (side effects and extrahepatic malignancy risk). Antiviral treatment indication is expected to be expanded, but it is necessary to begin with patients who are obviously at high risk. In this sense, patients in the fake IT phase may be suitable next targets. As reported in the accompanying article (Fig. 1), when a patient has any risk factors (e.g., age of >35 years, intermediate HBV DNA level [6–7 log10 IU/mL], and low platelet count), clinicians should heighten their suspicion if he or she is in the fake IT phase, a silent killer in disguise.
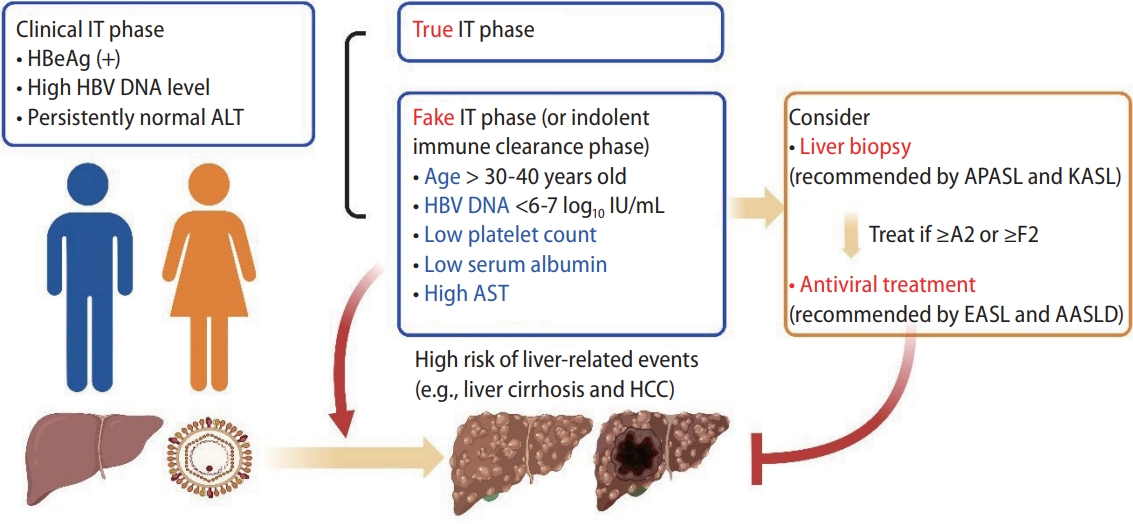
Clinical significance of fake IT phase. Patients in the fake IT phase had a higher risk of liver-related events (liver cirrhosis and HCC) than those in the true IT phase. Age, HBV DNA load, platelet count, and serum levels of albumin or AST can be used to suspect the presence of the fake IT phase. AASLD, American Association for the Study of Liver Diseases; ALT, alanine aminotransferase; APASL, Asian Pacific Association for the Study of the Liver; AST, aspartate aminotransferase; EASL, European Association for the Study of the Liver; HCC, hepatocellular carcinoma; IT, immune-tolerant; KASL, Korean Association for the Study of the Liver.
Notes
Authors’ contributions
Conceptualization, J-H Lee; Original draft, MH Hur and J-H Lee; Review and editing, MH Hur and J-H Lee.
Conflicts of Interest
MH Hur has no conflict of interest to disclose. J-H Lee receives research grants from Yuhan Pharmaceuticals and GreenCross Cell, and lecture fees from GreenCross Cell, Daewoong Pharmaceuticals, and Gilead Korea.
Abbreviations
AASLD
American Association for the Study of Liver Diseases
ALT
alanine aminotransferase
AST
aspartate aminotransferase
CHB
chronic hepatitis B
EASL
European Association for the Study of the Liver
HBV
hepatitis B virus
HCC
hepatocellular carcinoma
IT
immune-tolerant
TDF
tenofovir disoproxil fumarate
