KASL clinical practice guidelines for management of autoimmune hepatitis 2022
Article information
PREAMBLE
Purpose and scope
Autoimmune hepatitis (AIH) is an inflammatory liver disease of unknown etiology caused by an autoimmune mechanism. It can occur in all age groups and manifest as almost every type of liver disease, including asymptomatic liver enzyme elevation, acute hepatitis, acute liver failure (ALF), chronic hepatitis, or cirrhosis. There are no specific tests for diagnosing AIH, and diagnosis can be made by synthesizing several findings that are relatively characteristic of AIH. Immunosuppressive therapy based on glucocorticoids is the first-line treatment and is very effective for most patients. However, if the diagnosis is delayed or primary treatment is ineffective, serious complications, such as decompensated cirrhosis, hepatocellular carcinoma (HCC), and liver transplantation, could occur.
As AIH is a rare disease and responds well to first-line treatment, high-quality research on second-line treatments or specific situations is limited. Recommendations based on high-quality evidence are also limited, even in the U.S. or European guidelines. In South Korea, the prevalence of AIH is lower than that in the West, and research and awareness on AIH are lacking compared to viral hepatitis, which has a high disease burden. Moreover, South Korea still has no official treatment guidelines for AIH.
Therefore, we have systematically reviewed Korean and international studies to prepare appropriate guidelines based on evidence and to reflect domestic conditions as much as possible. In case related studies on clinically essential issues are lacking, we tried to present consensus opinions of experts. These guidelines have been developed through the reviews of medical evidence by experts to provide a practical reference for the treatment, research, and education of AIH. They are not absolute standards for treatment, and the best choice of practice for individual patients may vary depending on the individual circumstances. If relevant evidence based on new research results is accumulated in the future, these guidelines can be revised and supplemented. The guidelines cannot be modified, transformed, or reproduced without permission.
Target population
The target population of these guidelines include adults, adolescents, and pediatric patients with AIH.
Intended users
The following guidelines aim to provide clinical information useful for decision-making of healthcare providers involved in the diagnosis and treatment of AIH patients and to raise awareness of AIH among them, ultimately reducing morbidity and mortality and increasing the quality of life for AIH patients. In addition, these guidelines are intended to provide specific and practical information to resident physicians, practitioners, and trainers.
Guideline development group, process, and funding source
The Clinical Practice Guideline Committee for the Management of AIH (committee) was organized in accordance with proposals approved by the KASL Board of Executives. The committee consists of 12 hepatologists, one clinical pathologist, one pathologist, one pediatrician specializing in hepatology, and one methodology expert (Supplementary Table 1). All expenses were paid by KASL, and the financial support did not affect the independence of the contents of the guidelines. Each member of the committee collected, analyzed relevant evidence, and wrote the manuscript in his or her field. The timeline of the guideline development process is shown in Supplementary Table 2. Conflicts of interest among the members are summarized in Supplementary Table 3.
Literature search for evidence collection
The committee collected and analyzed relevant Korean and international literature through PubMed, MEDLINE, KoreaMed, KMBASE, RISS, and KISS to establish the guidelines based on the latest research and evidence. Only Korean and English literature were searched, and the search terms included “AIH” or “autoimmune liver disease” and specific terms of the subject.
Levels of evidence and grades of recommendations
The literature collected for evidence was analyzed through systematic review, and the levels of evidence were classified based on the revised Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) with modification (Table 1) [1-3]. They were categorized based on the possibility of changes in the assessment through further research as follows: high (A), with the lowest possibility; moderate (B), with certain possibility; and low (C), with the highest possibility. Specifically, depending on the type of study, randomized controlled trials start at a high level of evidence (A) and observational studies start at a low level of evidence (C). Considering factors affecting the study’s quality, the evidence level was raised or lowered further [2]. The strength of recommendation was suggested to be either strong (1) or weak (2), according to the GRADE system [4]. It was determined based on the clinical effects of recommendation, patient’s receptivity, and socioeconomic aspects, as well as the level of evidence. For example, a strong recommendation indicates that interventions could be applied in most patients with solid certainty in terms of a greater possibility of desirable effects, high-quality evidence, presumed patient-important outcomes, cost-effectiveness, preference, and compliance. A weak recommendation indicates a suggestion made with less certainty, which could be considered favorable for many patients. Alternative interventions could be chosen for “weak recommendations” according to the preferences of patients or medical practitioners.
List of key questions
The Clinical Practice Guideline Committee for the Management of AIH selected the following key questions and presented evidence and recommendations for them.
1. What are the incidence and prevalence of AIH?
2. What are the clinical features of AIH?
3. What are the characteristics of AIH type 1 and type 2?
4. What are the characteristics of overlap syndromes?
5. What are the concurrent autoimmune diseases of AIH?
6. How does AIH differ from AIH-like drug-induced liver injury (DILI)?
7. How is AIH diagnosed?
8. What autoantibody tests are required to diagnose AIH?
9. What are the characteristic histologic findings for AIH?
10. What are the diagnostic criteria for AIH, and what is the diagnostic usefulness of each criterion?
11. What are the proven non-invasive methods to assess liver fibrosis in AIH?
12. What should be evaluated before starting treatment for AIH?
13. Are pre-tests required before azathioprine (AZA) treatment for AIH?
14. What are the criteria for initiating immunosuppressive therapy for AIH?
15. What is the first-line treatment for AIH?
16. How is the treatment response for AIH evaluated?
17. What should be monitored during the immunosuppressive treatment for AIH?
18. What are the side effects of AIH treatment?
19. What are the criteria for terminating immunosuppressive treatment for AIH?
20. How are patients with AIH followed after the termination of immunosuppressive treatment?
21. How is recurrent AIH treated?
22. What is the second-line treatment for AIH?
23. What is the treatment for pediatric and adolescent patients with AIH?
24. What is the treatment for pregnant patients with AIH?
25. What is the treatment for elderly patients with AIH?
26. What is the treatment for overlap syndromes?
27. What is the treatment for AIH with non-alcoholic fatty liver disease?
28. What is the treatment for AIH accompanied by viral hepatitis?
29. What is the treatment for AIH which recurs or develops after liver transplantation?
30. What is the prognosis of AIH?
31. What are the complications of AIH?
32. What is the incidence of hepatocellular carcinoma in patients with AIH, and who is at high risk, and who needs surveillance?
In addition, the committee attempted to present evidence and recommendations by conducting a systematic review on the following topics: 1) Is there a difference between low-dose prednisolone with or without AZA and high-dose prednisolone with or without AZA in terms of efficacy and side effects as a first-line treatment for patients with AIH except acute severe AIH or hepatic failure?
Internal & external review, and approval process
Manuscripts and recommendations prepared by each member were reviewed for content integrity and validity of evidence through several meetings at the committee, and the quality of the guidelines was evaluated according to the criteria of AGREE II (Appraisal of Guidelines for Research and Evaluation II). The recommendations were assessed and revised based on the critical review by the Delphi Committee, consisting of 11 experts in the field of hepatology belonging to the KASL (Supplementary Table 4). The guidelines were reviewed at a meeting of an external review board, consisting of seven specialists in the field of hepatology, and at a symposium open to all KASL members and the public, and they were then further modified. The final manuscript was endorsed by the Board of Executives of KASL.
Release of the guideline and plan for updates
The KASL Clinical Practice Guideline for the management of AIH was released at the 6th Korea Digestive Disease Week 2022 (December 1, 2022) and will be published in Clinical and Molecular Hepatology. The Korean version of the guideline is available on the KASL website (http://www.kasl.org). The KASL plans to update the guidelines when new significant evidence is accumulated, and revision of the guidelines is deemed necessary to improve the national health of Korea.
EPIDEMIOLOGY
Incidence
According to a meta-analysis, the global annual incidence of AIH was 1.37 per 100,000 persons (95% confidence interval [CI], 0.95–18.0) in 2019. The regional annual incidence in Asia, Europe, and America were 1.31, 1.37, and 1.00 per 100,000 persons, respectively [5]. An analysis based on data from the rare and intractable disease registry program in the Korean National Health Insurance system presented that the age- and sex-adjusted AIH incidence rate in South Korea during 2011–2013 was 1.07 per 100,000 persons, which was similar to the global incidence. From 2009 to 2013, a total of 4,085 cases of AIH had been diagnosed, and the gender-adjusted annual AIH incidence rate was 0.31 per 100,000 in males and 1.83 per 100,000 in females. The incidence of AIH in females was 6 times that in males, and the mean age was 55 years (55 years in females, and 53 years in males). Age-specific incidence rate increased with age, and the peak age was 60s, with an annual incidence of 3.1 per 100,000 persons (Fig. 1A) [6]. While two peaks in incidence were shown in people in their late 10s and 50s to 70s in studies from Denmark, Sweden, and New Zealand [7-9], one peak was shown in the 60s age group in studies from United Kingdom and South Korea [6,10]. In a Danish study, the annual incidence of AIH increased from 1.37 per 100,000 persons in 1994 to 2.33 per 100,000 persons in 2012 [7], while the incidence did not increase in Sweden [8]. To date, there is no available data regarding the trend of AIH incidence in South Korea.
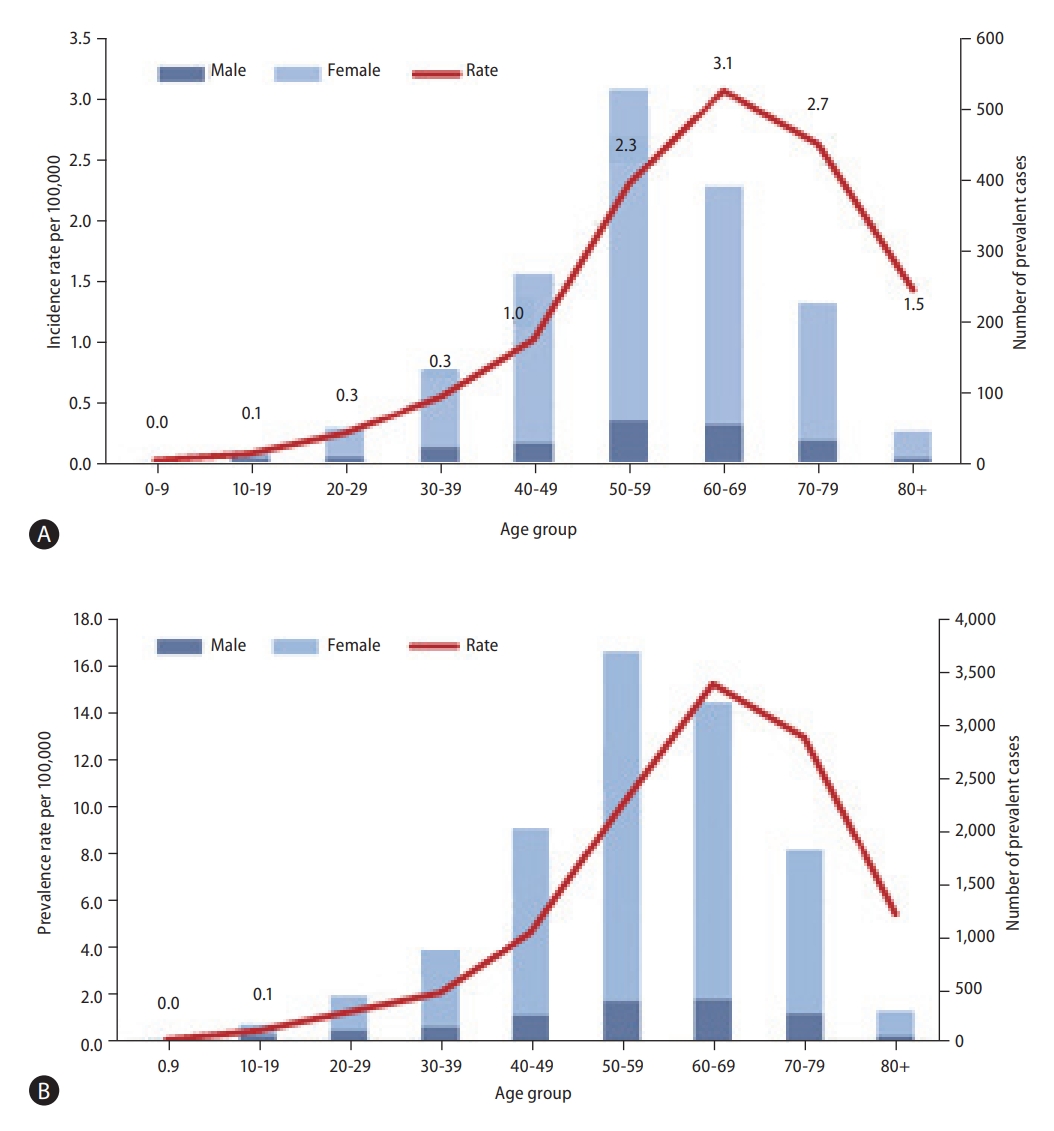
Epidemiology of autoimmune hepatitis in South Korea. (A) Average annual gender-adjusted incidence rate per hundred thousand population and incident cases (2011–2013) of autoimmune hepatitis by age (B) Average gender-adjusted prevalence rate per hundred thousand population and prevalent cases (2009–2013) of autoimmune hepatitis by age.
Prevalence
The global prevalence of AIH was 17.44 per 100,000 persons (95% CI, 12.01–22.87). The regional prevalence of AIH for Asia, Europe, and America was 12.99, 19.44, and 22.80 per 100,000 persons, respectively [5]. Meanwhile, according to a South Korean study including data from 2009 to 2013, the AIH prevalence was 4.82 per 100,000 persons and the gender-specific prevalence was higher in females, which was 8.35 per 100,000 persons in females, and 1.30 per 100,000 persons in males. The number of female patients was high among those in their 50s and 60s, and the peak prevalence for females was shown in their 60s (8.35 per 100,000 persons), and that for males was observed in their 70s (1.30 per 100,000 persons) (Fig. 1B) [6]. The prevalence was the highest among people in their 50s in New Zealand [9], and those in their 70s in Sweden and the United States [8,11]. Since 2000, the trend of prevalence increased in Sweden, New Zealand, and Japan [8,9,12]. The prevalence in South Korea increased from 3.9 per 100, 000 persons in 2009 to 5.76 per 100,000 persons in 2013, but further subsequent data are needed to evaluate the trend of AIH prevalence.
Genetic predisposition
It is well-known that human leukocyte antigen (HLA) DRB1*03 or DRB1*04 predisposes the onset of AIH and influences the natural course of the disease and treatment response [13-15]. In a study in South Korea, the frequencies of DRB1*0405 or DQB1*0401 were significantly increased in patients with AIH type 1 compared to the controls (odds ratio [OR], 3.74 & 3.95), and AIH type 1 was associated with the QRRAA motif at position 70-74 of the HLA-DRB1 molecule [16].
Summary
The annual incidence of AIH in South Korea was reported as 1.07 per 100,000 persons, and the prevalence was 4.82 per 100,000 persons. AIH occurred 6 times more frequently in females than in males. The incidence of AIH presented one peak among people in their 60s in South Korea, which was in contrast to a bimodal peak shown in those in their late teens and 60s in Western countries.
CLINICAL MANIFESTATIONS
AIH usually develops insidiously; however, the spectrum of symptoms and clinical manifestations are broad, ranging from asymptomatic to acute hepatitis, and AIH may also develop as fulminant hepatitis (Fig. 2) [17]. In addition, liver fibrosis has already progressed at the time of AIH diagnosis, and cirrhosis may be already present, or it may even appear as an acute exacerbation of cirrhosis [18]. In a Korean population study, 31–37% of patients were asymptomatic at the time of AIH diagnosis, and 13–32% of patients had cirrhosis as well [19]. Therefore, AIH should be considered as a differential disease in most liver diseases regardless of the degree of activity or fibrosis.
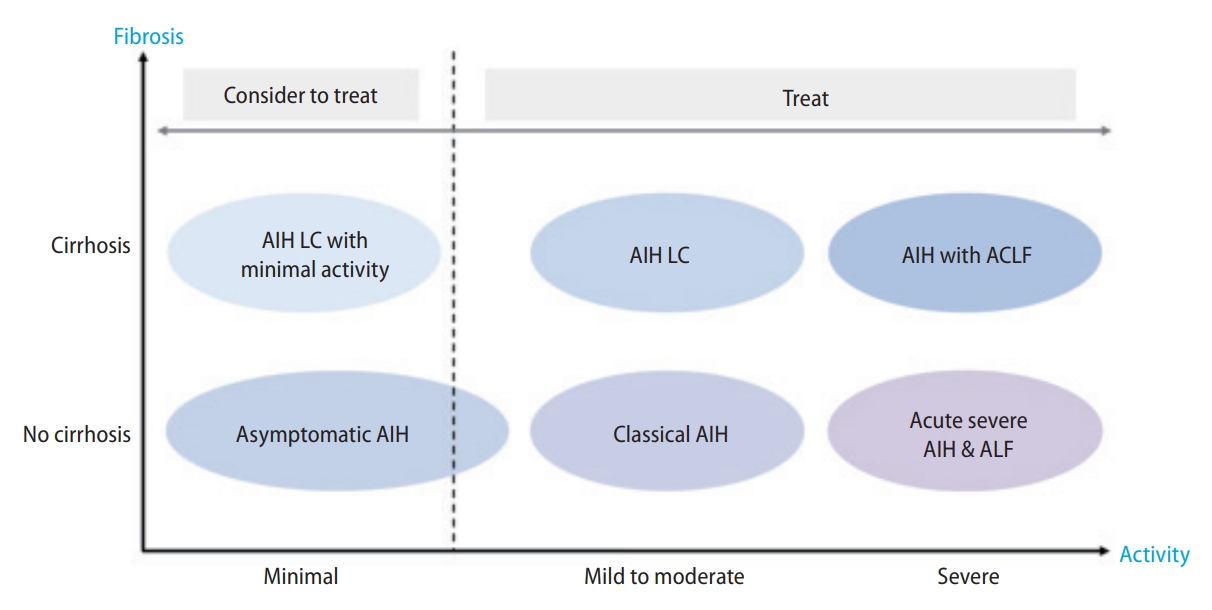
Clinical spectrum of autoimmune hepatitis. ACLF, acute on chronic liver failure; AIH, autoimmune hepatitis; ALF, acute liver failure; LC, liver cirrhosis.
Typical AIH presents as a form of chronic hepatitis with autoantibodies, hypergammaglobulinemia, and interface hepatitis in liver biopsy. Nonspecific fatigue is the most common. Loss of appetite, weight loss, muscle aches, joint pain, jaundice, and amenorrhea may be present, but low-grade fever and rash are less common [20].
Asymptomatic AIH
Patients who met the diagnostic criteria for AIH but showed no symptoms with elevated liver enzyme accounted for 25–37% of patients with AIH [19,21]. In patients with asymptomatic AIH, liver enzyme elevation may improve spontaneously; and in a previous study, symptoms appeared in 25.8% of the patients, and the average period until symptom onset was 2.00±2.46 years [22,23]. Compared to symptomatic AIH patients, asymptomatic AIH patients showed no difference in terms of age, sex ratio, disease progression, and histological findings, but they had significantly lower level of liver enzyme elevation and immunoglobulin G (IgG) [24]. In a Canadian study, asymptomatic AIH patients had no significant difference in 10-year survival compared to AIH patients with symptoms (80% vs. 83.8%, P=0.8) [22].
Acute severe AIH and acute liver failure
Acute severe AIH is defined as jaundice with a prothrombin time (PT) international normalized ratio (INR) of 1.5 to 2 but without hepatic encephalopathy due to AIH [21]. ALF with AIH was defined as a PT INR of 2 or greater or the development of hepatic encephalopathy within 26 weeks of AIH onset [21]. At the time of AIH diagnosis, about 25% of AIH patients showed acute presentation and 3–6% showed AIH with ALF [21,25]. Among patients with acute severe AIH, 29–39% of patients showed negative or weakly positive anti-nuclear antibodies (ANA) and 25-39% showed normal serum IgG [26,27]. In a recent study, heterogeneous hypo-attenuated region of the liver on non-contrast computed tomography (CT) scan was observed in 65% of AIH patients with ALF, whereas it was shown in only 2.2% of viral hepatitis patients with ALF [28]. These CT findings may be helpful for the diagnosis of AIH with ALF.
AIH with cirrhosis
Approximately 25–33% of AIH patients had liver cirrhosis (LC) at the time of AIH diagnosis regardless of clinical symptoms. Furthermore, it may present as decompensated cirrhosis or acute-on-chronic liver failure (ACLF) [18]. In a retrospective cohort study in the United States, the male gender, black or Hispanic race, older age (≥60 years), and lower education level were independent risk factors associated with cirrhosis at AIH diagnosis [29]. AIH patients with cirrhosis sometimes show burnt-out cirrhosis, in which histological characteristics of AIH are not observed. In such cases, the diagnosis of AIH can be made considering accompanying extrahepatic autoimmune diseases, the presence of autoantibodies, and past medical history [20,30]. In AIH patients with ACLF presentation, the proportion of ANA-negative was as high as 49%. Liver histology showed a moderate or high grade of interface activity in 90% and hepatic necrosis in 56% of the patients [31].
Type 1 and type 2 AIH
AIH can be classified into two types depending on the specific autoantibodies. Type 1 is characterized by the presence of ANA, smooth muscle antibody (SMA), and/or anti-actin antibody. Type 2 is characterized by the presence of antibody to liver kidney microsome type 1 (anti-LKM1) and/or antibody to liver cytosol type 1(anti-LC1), usually with the absence of ANA and SMA [21,32,33]. About 20% of patients with AIH may be negative for ANA, SMA, and anti-LKM1, even though they show clinical features of AIH. In such cases, antibody to soluble liver antigen (anti-SLA), an antibody test such as perinuclear antineutrophil cytoplasmic antibody (p-ANCA), can be additionally performed [21].
Type 1 AIH can occur at any age, but the onset peaks mainly around puberty and around the age of 60. Type 2 primarily occurs in children under 14 years of age or young adults, and is known to be very rare. A Korean study reported that anti-LKM1 was positive in about 1–3% of adult patients with AIH [19,34]. In a Korean single-center study of 14 pediatric patients with AIH, none of the patients were positive for anti-LKM1 [35]. Type 2 AIH is also known to be very rare in East Asian countries, such as Japan and Taiwan [34,36]. However, type 2 AIH is relatively common in South Asian countries, the United States, and Europe, and 13.2–16% of all pediatric patients with AIH have been reported as type 2 AIH in Malaysia and Canada [36-38]. In both types 1 and 2 AIH, IgG is often elevated but may be normal in the early stages of the disease, and sometimes normal or even lower in type 2 [37,39]. Type 1 AIH presents mainly in adults as acute or chronic non-specific symptoms such as fatigue, nausea, abdominal pain, and joint pain [40]. In type 2 AIH, acute onset occurs in 31–40% of the cases, and up to about 25% is known to develop in the form of ALF; and relatively many cases are unresponsive to treatment [21,32,41-43].
Autoantibody-negative AIH (seronegative AIH)
Autoantibody-negative AIH is defined as patients clinically and pathologically compatible with AIH, but without ANA, SMA, or anti-LKM; and accounts for 19–34% of AIH patients [21]. Even if autoantibodies are negative at the time of diagnosis, autoantibodies can become positive later in the course of the disease. In a retrospective cohort study, 60% of patients with autoantibody-negative AIH showed seroconversion up to 5 years of follow-up [44]. IgG4-related AIH, which showed high serum IgG4 levels and prominent IgG4-positive plasma cell infiltration in the liver, was 3.3–25% [45]. Autoantibody-negative AIH is diagnosed by clinical suspicion based on a diagnostic scoring system and the response to glucocorticoid treatment [46]. Autoantibody-negative AIH showed lower serum IgG level compared to autoantibody-positive AIH [47], and was relatively high at 29–39% in the AIH subgroup which presents as acute hepatitis or ACLF. Therefore, these patients are likely to be diagnosed with hepatitis of unknown etiology, and clinical suspicion and empirical treatment are essential for the diagnosis [27,31]. The 3-month biochemical response rate of autoantibody-negative AIH was 67–83%, which was similar to that of autoantibody-positive AIH [48].
Overlap syndromes
Overlap syndromes are defined as cases in which AIH is accompanied by other autoimmune diseases such as primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC), or IgG4-related cholangitis clinically, biochemically, serologically, and histologically [49].
AIH-PBC overlap syndrome
The prevalence of autoimmune hepatitis-primary biliary cholangitis (AIH-PBC) overlap syndrome was reported as 8–10% among AIH patients, and 7.4–11.7% in Korean studies [6,50,51]. In 8–12% of patients with AIH, antimitochondrial antibody (AMA) may be positive despite no histologic findings of bile duct damage or loss, and these patients respond well to glucocorticoid therapy alone [52]. Therefore, AMA positivity alone should not be diagnostic for AIH-PBC overlap syndrome. AIH-PBC overlap syndrome can be diagnosed simultaneously or sequentially. In a retrospective cohort study, 13.8% of the patients were diagnosed with AIH-PBC overlap syndrome, 7.8% were diagnosed with AIH and PBC simultaneously, 1.8% were diagnosed with AIH first, and 4.3% were diagnosed with PBC first [53].
AIH-PSC overlap syndrome
Adult patients with autoimmune hepatitis -primary sclerosing cholangitis (AIH-PSC) overlap syndrome are usually diagnosed first with AIH and then with PSC several years later [51]. AIH-PSC overlap syndrome can be suspected in AIH patients who have shown cholestatic liver biochemistry and insufficient response to immunosuppressive treatment. AIH-PSC overlap syndrome has been reported in 6-11% of AIH patients in the West; however, it is very rare in the East [54]. Patients with AIH-PSC overlap syndrome were younger (24 years old vs. 39.2 years old), and had higher levels of alkaline phosphatase [ALP]; 200.7 vs. 111.3 U/L) and bilirubin (2.7 vs. 1 mg/dL) at the time of diagnosis compared to patients with AIH alone [55].
Concurrent autoimmune diseases
About 14–44% of AIH cases are associated with other autoimmune diseases [56-58]. Autoimmune thyroid disease (AITD) is the most common concurrent autoimmune condition associated with AIH. Type 1 AIH is often associated with AITD, while type 2 AIH is generally associated with type 1 diabetes, AITD, and autoimmune skin diseases, such as vitiligo, leukocytoclastic vasculitis, urticaria, alopecia areata, etc [23,26,56-58] Other concurrent autoimmune conditions include rheumatoid arthritis (RA), mixed connective tissue disease, autoimmune hemolytic anemia (AIHA), idiopathic thrombocytopenic purpura, polymyositis, uveitis, Sjögren syndrome, systemic lupus erythematosus (SLE), and ulcerative colitis [59].
According to a recent report by South Korean investigators using the population-based National Health Insurance Service (NHIS) and the Rare Intractable Disease registration program between 2009 and 2013, the most common concurrent autoimmune disease was thyroid disorders, accounting for 10.5% of all cases among 3,783 patients with AIH. The second most common condition was SLE, accounting for 2.2%, followed by RA at 0.4% and systemic sclerosis at 0.2% [6].
In a small study on 205 North American adults diagnosed with AIH, concurrent extrahepatic autoimmune diseases occurred predominantly in women (85%) [60]. Co-occurring diseases varied by age. AITD, inflammatory bowel disease (IBD), and AIHA predominantly affected younger adults under the age of 30, while autoimmune thyroiditis and RA were more frequently observed among adults aged over 60 [60]. Furthermore, a small study on 86 North American adults diagnosed with AIH revealed that HLA DRB1*04:01-positive patients were more likely to have concurrent extrahepatic autoimmune diseases [13]. A questionnaire survey on 306 patients with AIH reported a higher prevalence of autoimmune disease in the first-degree relatives of patients than in the healthy controls (1,162 individuals; 55.9% vs. 35.7%) [61].
Autoimmune thyroid diseases
AITD is the most common concurrent autoimmune condition associated with AIH (10–23%). Hashimoto’s thyroiditis is associated with AIH, accounting for approximately 10.2–14.1% of all concomitant autoimmune diseases, followed by Grave’s disease at about 3–6% [58]. A retrospective study reported elevated IgG in patients with AIH accompanied by AITD [62].
Systemic lupus erythematosus
Approximately 2.8-3% of patients with AIH are accompanied by SLE [58,63]. A case report documented an occurrence of complications, such as myocarditis and thrombotic thrombocytopenic purpura, in an AIH patient with SLE [63]. On the contrary, 2.7–4.7% of patients with SLE were accompanied by AIH, and 19.4% of SLE patients with high liver enzyme levels were associated with AIH [64,65]. Moreover, 1.7% of SLE patients who received a biopsy due to suspected liver disease were found to have chronic hepatitis or LC [63,66]. A retrospective study reported that patients with AIH accompanied by SLE had higher IgG levels, and those with higher IgG had a poor prognosis [67].
Sjögren syndrome and rheumatoid arthritis
Sjögren syndrome is observed in about 2.8–7% of patients with AIH [58,68]. The association between Sjögren syndrome and liver disease was first reported in 1954 [69]. The prevalence of AIH among patients with Sjögren syndrome is not yet clearly known, with prevalence estimates ranging widely from 4 to 47% [70]. RA develops in approximately 2–4% of patients with AIH [58,68,71]. Immunosuppressive therapy is a favorable treatment option for RA and prevents joint deformity [71]. RA more commonly occurs in older AIH patients than in younger AIH patients [68,71].
Inflammatory bowel disease
IBD occurs in about 2–11.4% of AIH patients [72-75]. AIH is observed in approximately 3.7–11.4% of IBD patients [58,73]. In particular, ulcerative colitis is primarily associated with PSC, but AIH can also occur in 2–8% of AIH patients [71]. When proctoscopic examination was performed annually in 105 AIH patients receiving glucocorticoid treatment, ulcerative colitis was detected in 12 patients (11.4%) [73]. Meanwhile, patients with AIH were less likely to develop Crohn’s disease at a frequency of 1–6% [71]
In addition to the conditions mentioned above, multiple sclerosis occurs in about 0.17% of AIH patients, and this proportion is higher than the 0.02% prevalence of the general population [76,77]. Furthermore, AIH can also be rarely associated with leukoplakia, alopecia areata, celiac disease, type 1 diabetes mellitus, idiopathic thrombocytopenic purpura, pulmonary fibrosis, Raynaud’s phenomenon, etc.
Summary
AIH is usually manifested in the form of chronic hepatitis, but it can also manifest in the form of various liver diseases, such as asymptomatic, acute hepatitis, acute severe hepatitis including fulminant hepatitis, cirrhosis, and acute exacerbation of cirrhosis.
AIH can be divided into type 1 (ANA, SMA) and type 2 (anti-LKM1, anti-LC1) based on specific autoimmune antibodies, and type 2 AIH is very rare in South Korea.
A variety of autoimmune diseases accompany AIH patients, and AITD is the most common type.
DIAGNOSIS
Diagnostic criteria
The diagnosis of AIH is based on the characteristic clinical and laboratory findings (elevated serum aspartate aminotransferase [AST], alanine aminotransferase [ALT] and increased IgG concentration), the presence of characteristic autoantibodies, and compatible histological abnormalities (Figs. 3, 4). AIH lacks a signature diagnostic marker, and the diagnosis requires the exclusion of other diseases (viral hepatitis, alcoholic liver disease, non-alcoholic steatohepatitis, DILI, Wilson’s disease, hereditary hemochromatosis, etc.) [18,21,78].
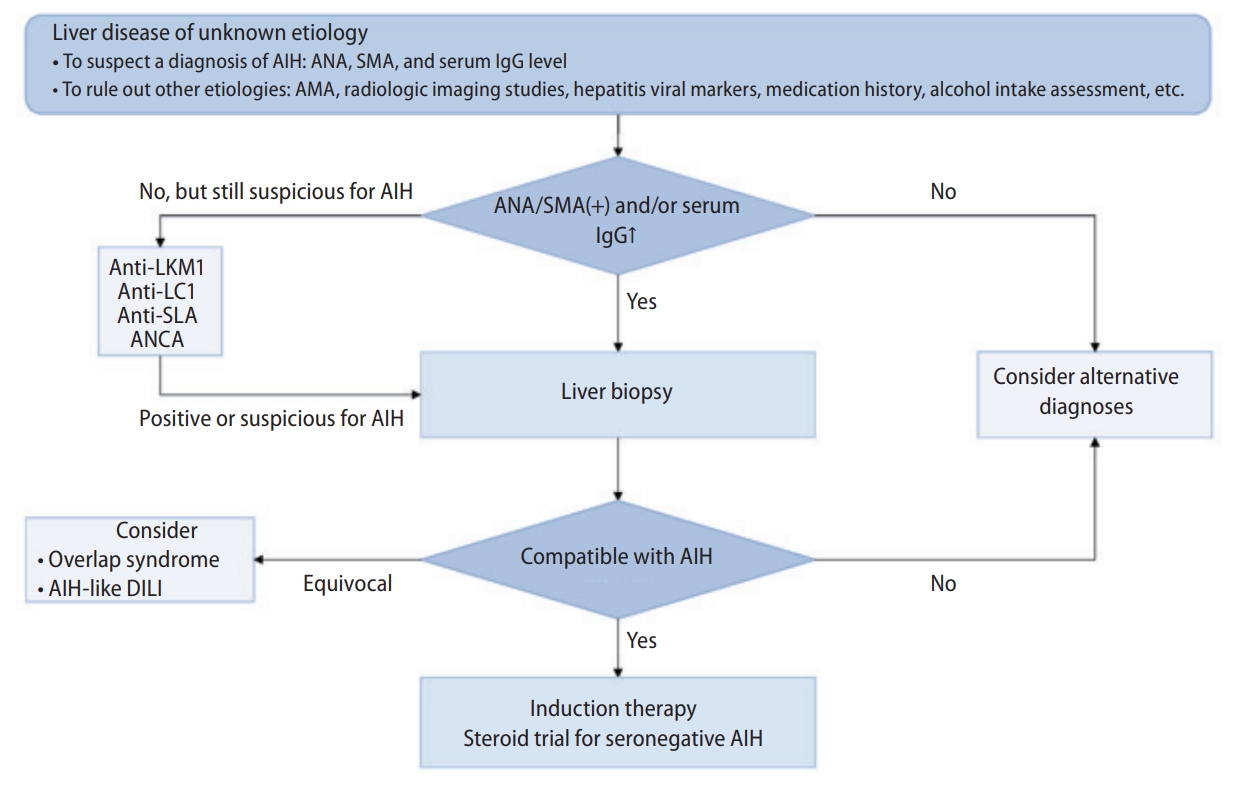
Diagnostic algorithm of autoimmune hepatitis. AIH, autoimmune hepatitis; AMA, antimitochondrial antibody; ANA, antinuclear antibody; ANCA, antineutrophil cytoplasmic antibody; anti-LC1, antibody to liver cytosol type 1; anti-LKM1, antibody to liver kidney microsome type 1; anti-SLA, antibody to soluble liver antigen; DILI, drug-induced liver injury; IgG, immunoglobulin G; SMA, smooth muscle antibody.
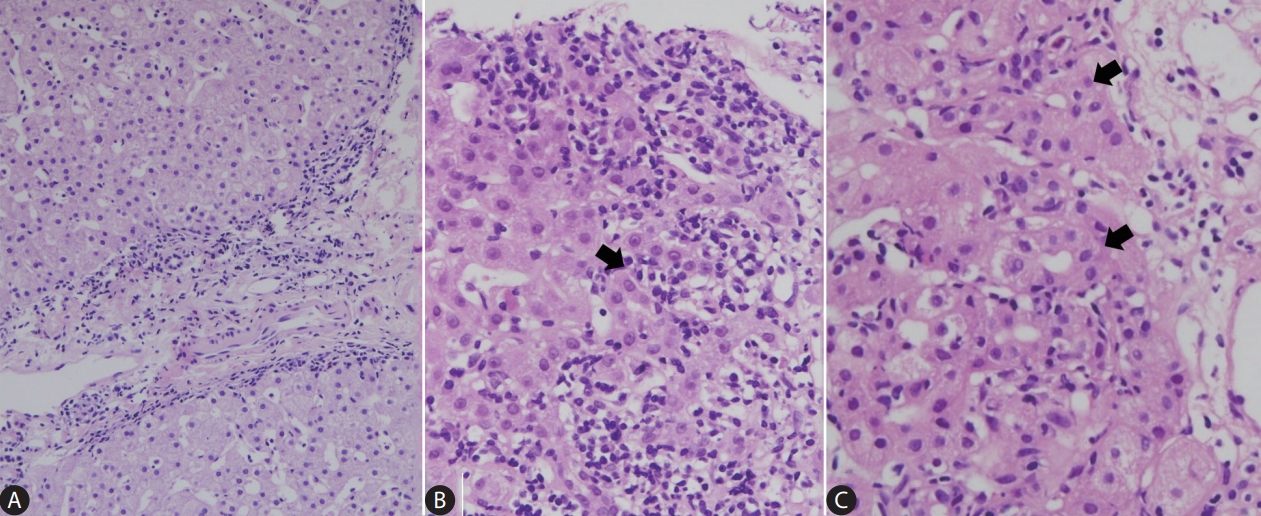
Histopathology of autoimmune hepatitis. (A) The microscopic features of autoimmune hepatitis. Mononuclear cells, including lymphocytes and plasma cells, are seen at the interface between the portal tract and hepatic lobule (H&E stain, x100). (B) Clusters of plasma cells (arrow) are often seen at high-power magnification (H&E stain, x400). (C) Hepatocyte rosettes (arrows) are shown (H&E stain, x400).
Although serum bilirubin and aminotransferase are markedly raised, normal or mildly elevated cholestatic enzymes are characteristic of AIH. The elevation of serum IgG level is a common feature, but IgA and IgM levels are usually normal [78-81]. In a European multicenter study, IgG was normal in about 10% of the patients; and even in these patients, clinical features were similar to typical AIH [82]. IgG was normal in 25–39% of AIH with acute presentation, according to studies from Japan [26,27,83].
Liver biopsy, which is an essential procedure in the diagnosis of AIH, was done in 54–75% of the Korean AIH patients [19]. Liver histology is important not only in confirming the clinical diagnosis of AIH, but also in differential diagnosis of AIH. Liver biopsy is considered a prerequisite for the diagnosis of AIH. The general view is that AIH cannot be diagnosed without compatible histological findings, considering the differentiation from other diseases and the discrimination of overlapping syndromes, although there is an opinion that a biopsy may not be necessary if the laboratory features are sufficiently typical [18,21,26,78,84]. Therefore, liver biopsy is essential if there are no contraindications.
Autoantibodies
Autoantibody ANA and SMA are used as screening tests for AIH [85]. Anti-LKM1, anti-LC1, anti-SLA, and ANCA can also be tested if ANA and SMA are negative. HEp-2 cells for ANA and rodent tissues for SMA are used as the target antigens in indirect immunofluorescence assays (IFA), which are the primary methods for detecting ANA and SMA (Table 2). Multiple autoimmune liver disease antibodies can be evaluated simultaneously by immunoblot for anti-LKM1, anti-LC1, and anti-SLA. The conventional serum dilution that tested positive for ANA and SMA using the IFA method is 1:40 for adults and 1:20 for children, and it is 1:10 for anti-LKM1 and anti-LC1. Repeat testing may be necessary for an appropriate diagnosis and classification if the initial autoantibody test is negative.
Since ANA targets antigens whose specificity has not been determined, testing using enzyme-linked immunosorbent assay (ELISA) can result in false negatives in about one-third of the patients [86]. Actin is one of the cytoskeletal antigens that SMA reacts to, and anti-actin is found in about 40% of cases [20]. When both ANA and SMA are detected, the diagnostic value would be high [20]. Anti-SLA has diagnostic relevance as it is the sole disease-specific autoantibody for AIH [87]. However, a solid phase immunoassay test, such as ELISA or immunoblot, should be carried out since it cannot be detected by the IFA method. The serological markers of type 2 AIH, anti-LKM1 and anti-LC1 should be ruled out as they can be found in 5–10% of adult and pediatric patients with chronic HCV infection. The ANCA test of the IFA method can be used when the results of the ANA, SMA, and anti-SLA tests are negative. In some patients with type 1 AIH, perinuclear anti-neutrophil nuclear antibody (p-ANNA) or p-ANCA may be the only serological markers [88,89]. AMA, a serological marker specific to PBC, is performed for the differential diagnosis of overlap syndrome and can be detected in 8–12% of patients with typical AIH [52,90]. Autoantibody titers in pediatric patients can be useful biomarkers reflecting disease activity and may also be useful for monitoring treatment response. Serology laboratories and physicians need to increase their expertise and communicate closely in interpreting the autoimmune liver disease serology to provide maximum benefits to patients. If the diagnosis is uncertain, it is necessary to refer a serological test to a specialized reference laboratory for a complete evaluation.
Histological findings
Histopathologically, the typical AIH case demonstrates a hepatitic picture with portal lymphoplasmacytic infiltration and interface hepatitis (Fig. 4) [81,91-93]. Plasma cells are often abundant. Various degrees of lobular necroinflammation have been observed. Fibrosis typically begins from the portal tracts and eventually progresses to cirrhosis. Periportal hepatocytes often appear in rosette configuration (hepatocytic rosettes), and emperipolesis may also be seen. AIH may demonstrate an acute hepatitis pattern on histology, characterized by prominent lobular necroinflammation and zone 3 confluent necrosis, with relatively mild or minimal portal changes [93-95]. In addition, fibrosis may be absent in the earlier stages of AIH. Once AIH progresses to cirrhosis, the typical histological features, such as portal inflammation and interface activity, may become inconspicuous (so-called “burnt out” AIH); and at this stage, it is often difficult to distinguish AIH-cirrhosis from cirrhosis of other etiologies. Bile duct injury is not a typical feature of AIH, and if marked bile duct injury is seen in a background of otherwise typical AIH, the possibility of overlap syndrome (AIH-PBC, AIH-PSC) may be entertained. Drug/toxin-induced liver injury may present with AIH-like histology; and therefore, it is always important to clinically exclude this possibility.
According to the 1999 revised scoring system and the 2008 simplified system, the presence of portal lymphoplasmacytic infiltration, interface hepatitis, hepatocytic rosettes, and emperipolesis are the key histological features for a diagnosis of AIH [81,91]. However, these staging systems have some limitations: hepatocytic rosettes and emperipolesis are not specific for AIH, as they may be seen in the setting of severe hepatocyte injury and regeneration of any etiology; and AIH with acute hepatitis patterns are less likely to qualify as definite AIH with these scoring systems due to the lack of portal changes on histology [92,93,96]. Recent consensus documents suggest that the possibility of AIH could be suggested for cases with less than mild portal changes, if there is at least moderate lobular necroinflammation and other etiologies have been sufficiently excluded [92,93].
For the diagnosis and staging of AIH, it is important that a sufficient number of portal tracts are included in the liver biopsy sample. It is recommended that the biopsied tissue is at least 1.5 cm in length and that wider cores are obtained to ensure evaluation of the entire circumference of the portal tract [97-99]. In order to accurately evaluate the degree of fibrosis, collagen stains, such as Masson’s trichrome, are necessary in addition to the routine hematoxylin-eosin stains.
Diagnostic scoring sytems
A diagnostic scoring system was proposed by the International Autoimmune Hepatitis Group (IAIHG) to help diagnose atypical cases as well as typical cases of AIH, quantify diagnoses, and enable objective comparison. In 1999, a revised original scoring system was announced, and in 2008, a simplified scoring system was also developed (Tables 3, 4) [81,91,100]. In South Korea, diagnoses were also based on the revised original scoring system and simplified scoring system [19]. The revised diagnostic scoring system is known to help diagnose patients with complex or atypical features, whereas the simplified scoring system is more accurate in typical patients [101]. In a Japanese study, the revised scoring system showed 100% sensitivity and 93% specificity, and the simplified scoring system showed 85% sensitivity and 99% specificity [102]. In a Korean study, the diagnostic sensitivity and positive predictive value of the simplified criteria compared with the revised original criteria were 69.9% and 86.4%, respectively [34,103]. Therefore, considering the high sensitivity of the revised scoring system and the high specificity of the simplified scoring system, if the score calculated by the simplified scoring system is low, reassessment with the revised scoring system should be considered [101].
The revised diagnostic scoring system can be applied to pediatric patients, but it should be noted that the autoantibody titer of children is lower than that of adults [91]. The simplified scoring system provides a moderate sensitivity, but it may be helpful in the diagnosis of pediatric AIH [104].
Since this diagnostic scoring system was not devised for Asian countries, including South Korea, there is insufficient evidence for the association and weighting of each item with diagnosis targeting Koreans. In particular, in a single Korean study on HLA types among genetic predispositions, the frequency of HLA DRB1*0405 and DQB1*0401 was high in type 1 AIH patients, while HLA DRB1*03 showed no association with AIH, limiting the application of the existing revised scoring system. Therefore, in the future, it is necessary to improve the items on genetic predisposition applicable to Koreans through further research on HLA types related to AIH in the Korean population [16].
Overlap syndromes
AIH-PBC overlap syndrome
The “Paris criteria” is the most common and effective method to diagnose the AIH-PBC overlap syndrome [105]. It requires at least two of the following three diagnostic criteria for each disease. Two of the following three criteria for PBC should be met: (1) serum ALP level ≥2-fold the upper limit of normal (ULN) range or serum gamma-glutamyl transferase (GGT) level ≥5-fold ULN; (2) presence of AMA; and (3) a liver biopsy specimen showing florid bile duct lesions (non-suppurative destructive cholangitis of interlobular bile duct). For AIH, it requires two of the following three diagnostic criteria: (1) ALT ≥5-fold ULN; (2) serum IgG level ≥2-fold ULN or presence of SMA; and (3) a liver biopsy with moderate or severe interface hepatitis [106]. Since the revised diagnostic scoring system excludes PBC, a Korean study was performed to demonstrate that the simplified diagnostic scoring system can help diagnose overlap syndrome; however, due to the small sample size of the study, it had limited clinical application [107].
AIH-PSC overlap syndrome
Criteria for the diagnosis of AIH-PSC overlap syndrome include the presence of typical features of AIH, absence of AMA, and evidence of large duct PSC based on bead-like appearance characterized by focal narrowing and dilatation of the bile duct on magnetic resonance cholangiopancreatography (MRCP) or endoscopic retrograde cholangiography (ERCP), or evidence of small-duct PSC based on characteristic fibrous obliterative cholangitis on histology [108]. In a study of patients with PSC, it was reported that the revised diagnostic scoring system could help diagnose AIH-PSC overlap syndrome [109].
[Recommendations]
1. AIH is diagnosed by excluding liver injury from other causes and integrating laboratory findings (increased serum AST, ALT, and/or IgG), the presence of autoantibodies, and compatible histologic findings. (B1)
2. If AIH is suspected, ANA and SMA are performed as screening tests. (B1) Anti-LKM1, anti-LC1, anti-SLA, or ANCA can be further examined if clinically necessary. (C1)
3. AIH can be diagnosed with a revised diagnostic scoring system or a simplified diagnostic scoring system. (B2)
4. If a patient with AIH shows a cholestatic pattern of liver function test, AMA and cholangiography should be performed, considering the possibility of AIH-PBC overlap syndrome or AIH-PSC overlap syndrome. (C1)
AIH-like drug-induced liver injury
Clinical manifestations and pathological findings of DILI caused by an unpredictable idiosyncratic drug reaction or hypersensitive drug reaction were similar to those of AIH in about 2-17% of patients reported as AIH [110-112]. The leading causative agents are known to be nitrofurantoin, minocycline, alpha-methyl DOPA, and hydralazine [113]. DILI can resemble the clinical manifestations of AIH by causing the formation of serum autoantibodies and gammaglobulinemia. A liver biopsy can be performed to differentiate between DILI and AIH. However, DILI may be difficult to distinguish from AIH due to the manifestation of interface hepatitis and plasma cell infiltration [114]. Therefore, drugs and supplements exposed before disease onset should be clearly identified. After drug exposure, the latency period of AIH-like DILI varies greatly, ranging from 1–8 weeks to 3–12 months [21,115]. Moreover, the assessment of response to and recurrence after glucocorticoid therapy is helpful in differentiating between AIH and DILI [113].
AIH-like DILI mainly affects women, and acute hepatitis is the common manifestation of DILI in more than 60% of all cases. Cardinal symptoms include nausea, vomiting, lethargy, and right upper quadrant pain. Approximately 30% of DILI patients display signs of drug hypersensitivity reactions, such as fever, rash, and increased eosinophils [21]. In genetic tests, HLA DRB1*03:01 or HLA DRB1*04:01 are found to be similar to healthy controls, and autoimmune disease is rarely accompanied [116]. When a previous study reviewed 261 patients diagnosed with AIH over a decade, AIH-like DILI was detected in 24 patients, accounting for 9.2% of all cases [110]. The median age was 53 years (interquartile range, 24–61), and nitrofurantoin and minocycline were the most common agents associated with DILI [110]. Liver enzyme levels were elevated up to 5–20 times the normal amount, while ALP increased slightly. Serum bilirubin varied to over 20 mg/dL from the normal range, and elevated gamma globulin levels, ANA positivity (83%), and SMA positivity (50%) were observed [110]. Furthermore, the symptoms were mitigated by stopping the causative drug and receiving glucocorticoid treatment. No relapse was observed after discontinuation of immunosuppressive treatment, and none of the patients progressed to LC [110]. Immune-related adverse events are being increasingly reported, with the growing use of immune checkpoint inhibitors that activates immune cells to block various cancers. DILI caused by immune checkpoint inhibitors is highly responsive to glucocorticoid therapy, and it usually shows negative or low levels of serum ANA and SMA and has normal gamma globulin levels [117].
Monitoring is warranted to check for improvement in clinical manifestations and laboratory findings without recurrence after stopping the causative agents. Most cases of DILI improve within a month, but may rarely persist for more than 3 months. According to Hy’s Law criteria, when serum AST and ALT levels are elevated more than three times the ULN and serum bilirubin is greater than two times the ULN, it leads to the risk of death or liver transplantation in approximately 9–12% of cases [118,119]. The use of glucocorticoids is considered when symptoms show no improvement despite the suspension of drug use and meet Hy’s Law criteria. The diagnosis of DILI can be confirmed when normal values are maintained in routine blood tests after the withdrawal of glucocorticoid therapy. On the contrary, repeatedly elevated liver enzymes may indicate AIH. Relapsing hepatitis is managed the same way as AIH using immunosuppressive agents [120]. Most patients with AIH-like DILI have a good prognosis, but this condition may rarely result in death, in about 5%, due to idiosyncratic drug response and require liver transplantation in about 4.5% of cases [121].
Summary
AIH-like DILI is challenging to distinguish from AIH only based on clinical manifestations, laboratory, and biopsy findings. This condition can be discriminated from AIH based on the history of medication before disease onset and no recurrence despite discontinued steroid treatment.
Non-invasive fibrosis assessment
Serum biomarkers
The degree of liver fibrosis of AIH patients can be estimated using serum panel. The serum panel including FibroTest which combines five serum biochemical markers (α-macroglobulin, apolipoprotein A1, haptoglobin, total bilirubin, GGT) with patient age and gender [122-124], aspartate aminotransferase-to-platelet ratio index (APRI) [125], Fibrosis-4 index (FIB-4) which combines patient age with measurements of 3 biomarkers (AST, ALT and platelet) [126,127], enhanced liver fibrosis test (ELF) which combines tissue inhibitor of metalloproteinases 1 (TIMP-1), amino-terminal propeptide of type III procollagen (PIIINP) and hyaluronic acid (HA) [128,129], are well-validated non-invasive tests in viral hepatitis. However, their role in predicting the progression of liver fibrosis, long-term prognosis, and development of hepatocellular carcinoma (HCC) in AIH remains unknown [130]. Another serum marker Mac-2-binding protein glycan isomer (M2BPGi) has been studied in Japanese AIH patients, but its applicability in Korean patients requires further validation [131,132].
Imaging modalities
The ultrasound-based measurements of liver stiffness comprise transient elastography (TE), 2D shear wave elastography (SWE), and point SWE, while other methods include magnetic resonance elastography (MRE).
In AIH, studies based on FibroScan® are relatively more common than other non-invasive imaging modalities [133-136]. The median value of liver stiffness measurement (LSM) in AIH patients was higher than that of healthy controls (11.2±8.2 kPa vs. 4.3±1.4 kPa, P<0.01) [136]. A robust positive correlation was observed between LSM and histological fibrosis stage [134]. However, the LSM value was higher within 3 months of treatment, and the area under the receiver operating characteristic curve (AUROC) at 6 months was higher than that at 3 months of treatment (≥F2, 0.68 vs. 0.97; ≥F3, 0.8 vs. 1.0; F4, 0.71 vs. 1.0). The best cut-off values for ≥F2, ≥F3, and F4 at 6 months of treatment were 5.8 kPa, 10.5 kPa, and 16.0 kPa, respectively [134]. Since hepatic inflammation impacts LSM, ≥F3 can be more accurately diagnosed after 6 months of treatment when hepatic inflammation is resolved [134]. In another retrospective study, patients who failed to achieve complete biochemical remission showed a slight increase in LSM (+1.7%/year; 95% CI, -6.0% to 12.1%; P=0.19), while a significant decrease in LSM (-7.5%/year; 95% CI, -11% to -2.0%; P=0.0003) was observed in the complete biochemical remission group, indicating that fibrosis regression can be monitored by TE [135]. According to a meta-analysis, TE performed better than serum markers, FIB-4 and APRI, in staging advanced fibrosis ≥F3 [130,137].
The AUROCs for 2D-SWE in diagnosing ≥F2, ≥F3, and F4 were 0.85, 0.85, and 0.86, respectively [138]. Other studies reported similar AUROCs ranging from 0.781 to 0.84 [139,140], showing higher predictive efficacy compared to APRI and FIB-4 (AUROC, 0.84 vs. 0.57 vs. 0.63) [140]. A single-center study in South Korea on point SWE which generates shear wave in one area revealed an AUROC of 0.8, similar to the results of studies from other countries that showed point SWE outperforming APRI and FIB-4 [141].
The MRE has the advantage of evaluating the whole liver, and MRE appeared to outperform TE for staging hepatic fibrosis in some studies focusing on other liver diseases [142]. One study based on 36 patients showed that the AUROCs for advanced fibrosis (≥F3) and cirrhosis (F4) were 0.97 and 0.98, respectively [143]. The best cut-off values for ≥F3 and F4 were 4.1 kPa (sensitivity 89.5%; specificity 100%) and 4.5 kPa (sensitivity 92.5%; specificity 96%), respectively, revealing a very high diagnostic accuracy [143]. Although a study comparing MRE with TE is lacking, MRE outperformed APRI and FIB-4; and therefore, further evaluation on the role of MRE in AIH patients is required in the future.
[Recommendations]
1. Transient elastography can be useful in diagnosing advanced fibrosis (≥F3) or cirrhosis in patients with AIH and should be performed after hepatic inflammation has been resolved in patients undergoing induction therapy. (C2)
TREATMENT
Treatment aims and indications
Treatment aims and definitions of treatment endpoints
The goals of AIH treatment are to minimize the risk of complications caused by drugs, control the liver inflammation, and achieve remission to suppress the progression of liver disease. To achieve these aims, long-term or permanent maintenance therapy after remission is required in most patients with AIH.
The ideal biochemical response is the normalization of serum ALT, AST, and IgG, and the ideal treatment response is the loss of histologic inflammation along with the biochemical response [21,93,144-148]. Even with the biochemical response, histologic inflammation often persists. Since aminotransferases and IgG do not reflect the activity of histologic inflammation, especially in the case of cirrhosis [82,149], liver biopsy may be necessary to confirm the loss of histologic inflammation. A study with paired liver biopsy in 120 patients with a biochemical response for more than 6 months showed that 46% of patients had a histological activity with an Ishak score of 4 or higher, and it was an independent factor associated with death or liver transplantation [149].
Complete biochemical response is the normalization of serum transaminases and IgG below the ULN within 6 months of treatment (Table 5) [150]. Insufficient response is the lack of complete biochemical response, determined no later than 6 months after the initiation of treatment. Non-response is <50% decrease of serum transaminases within 4 weeks after the initiation of treatment. Remission refers to a case where the hepatitis activity index (HAI) of liver tissue is less than 4 out of 18 points [150]. Intolerance to treatment is any adverse event possibly related to treatment as assessed by the treating physician, leading to potential discontinuation of the drug [21].

Endpoints for AIH treatment as proposed by the International Autoimmune Hepatitis Working Group after a consensus process
Persistent elevation of AST or ALT level during treatment is known to predict the progression of liver diseases and poor prognosis, such as recurrence, histological activity, cirrhosis, and hepatocellular carcinoma, after treatment is discontinued [146-148,151,152]. According to a retrospective study of 132 patients with AIH, patients whose serum biochemical indicators did not return to normal had a 3–11 times higher risk of relapse after discontinuation of treatment compared to patients with normal serum biochemistry [146]. Another study reported that only 4% of patients with normal serum biochemical indicators experienced histological deterioration, while 54.5% of patients without normalization experienced histological and clinical deterioration [151]. In addition, since serological indicators, especially ALT and IgG, are closely related to histological activity [153], normalization of these can be used as indicators to predict histological remission.
Treatment indications
Active research on the treatment of AIH was conducted from the 1960s to the 1970s [154-158], and treatment regimens based on the results of these studies are valid to date. Since the hepatitis C virus was discovered in 1989, there is a possibility that chronic hepatitis C patients may have been included among patients diagnosed with AIH before 1989, and therefore, some hepatitis C patients may have been included in the initial clinical trial. In a prospective randomized controlled study conducted for the first time in patients with chronic active hepatitis, the placebo group without treatment showed a high mortality rate of 56% at the 72-month follow-up, whereas the mortality rate of patients treated with prednisolone decreased to 14% [154]. In several subsequent randomized controlled studies, patients with chronic active hepatitis who were not treated showed a high mortality rate (41% at 3–3.5 years of follow-up), whereas those treated with prednisone alone or prednisone plus AZA showed a reduced mortality by 6–10% [155,157]. Accordingly, it has been confirmed that untreated active AIH has a very poor prognosis, and that appropriate immunosuppressive therapy improves liver function and increases survival.
Evidence on the natural course and benefits of immunosuppressive treatment in asymptomatic AIH patients with mild inflammatory activity is still insufficient. A Canadian single-center cohort study of 126 patients with AIH reported a 10-year survival rate of 80.0% (95% CI, 62.5–97.5%) for patients with asymptomatic AIH, and untreated asymptomatic patients showed a statistically insignificant survival difference compared to asymptomatic patients who received treatment [22]. On the other hand, in another retrospective study conducted in the United States, some asymptomatic patients with mild activity reached remission without treatment, but the rate of reaching remission was significantly lower than that of patients who received immunosuppressive therapy (12% vs. 63%, P= 0.006), and their 10-year survival rate was also significantly lower (67% vs. 98%, P<0.01) [159]. AIH may have reached remission spontaneously without treatment, but the spontaneous remission did not persist after recurrence [22,159]. In a large retrospective study of 305 patients with AIH, asymptomatic patients had significantly lower biochemical and histological activity compared to symptomatic patients, but the response rate to immunosuppressive therapy (complete response rate P=0.558; non-response rate P=0.462) and liver-related prognosis (P=0.975) were found to be similar between the two groups [24]. If AIH is not treated, it is difficult to predict the disease course as the activity continuously changes; and a significant number of asymptomatic patients develop symptoms (25.8–69.6%) [22,160], experience liver disease progression (22.2–50%) [24,159,160], or progress to hepatocellular carcinoma, end-stage liver disease, or liver failure [159,160]. In a multicenter longitudinal cohort study in the UK, all patients with AIH treated with glucocorticoids had lower overall mortality and lower liver transplant rates compared to untreated patients (hazard ratio [HR], 0.25; 95% CI; 0.14–0.45; P<0.001); and in particular, the overall mortality and liver transplantation rates were significantly lower (HR, 0.13; 95% CI, 0.04–0.42; P=0.001) when even asymptomatic patients were treated [161].
Considering the natural course of AIH and the effect of immunosuppressive therapy, active AIH patients with abnormal clinical and laboratory findings (elevation of AST, ALT, and IgG) or liver tissue findings suggesting intrahepatic inflammation (HAI ≥4) are subject to immunosuppressive treatment. When treatment is withheld in asymptomatic inactive patients with an HAI score of less than 4 without advanced fibrosis, liver enzyme levels and IgG markers should be monitored regularly.
[Recommendations]
1. The goal of AIH treatment is to achieve remission by controlling the liver inflammation, thereby suppressing the progression and complications of liver disease. (B1)
2. Patients with active AIH should be treated with immunosuppressive therapy. (A1) When treatment is withheld in asymptomatic inactive patients with an HAI score of less than 4 without advanced fibrosis, liver enzyme levels and IgG markers should be monitored regularly. (C1)
3. In patients with AIH, serum aminotransferase levels and IgG are measured regularly to evaluate treatment response after initiation of treatment. (B1)
First-line treatments
First-line standard therapy
For the induction of remission of AIH, prednisolone 20–40 mg and AZA 50–150 mg are administered in combination daily, or prednisolone 40–60 mg alone daily (Fig. 5). Combination therapy of prednisolone at higher doses (up to 1 mg/kg/day) and AZA can induce rapid remission in patients with AIH without cirrhosis [162], but steroid-related side effects should be taken into consideration.
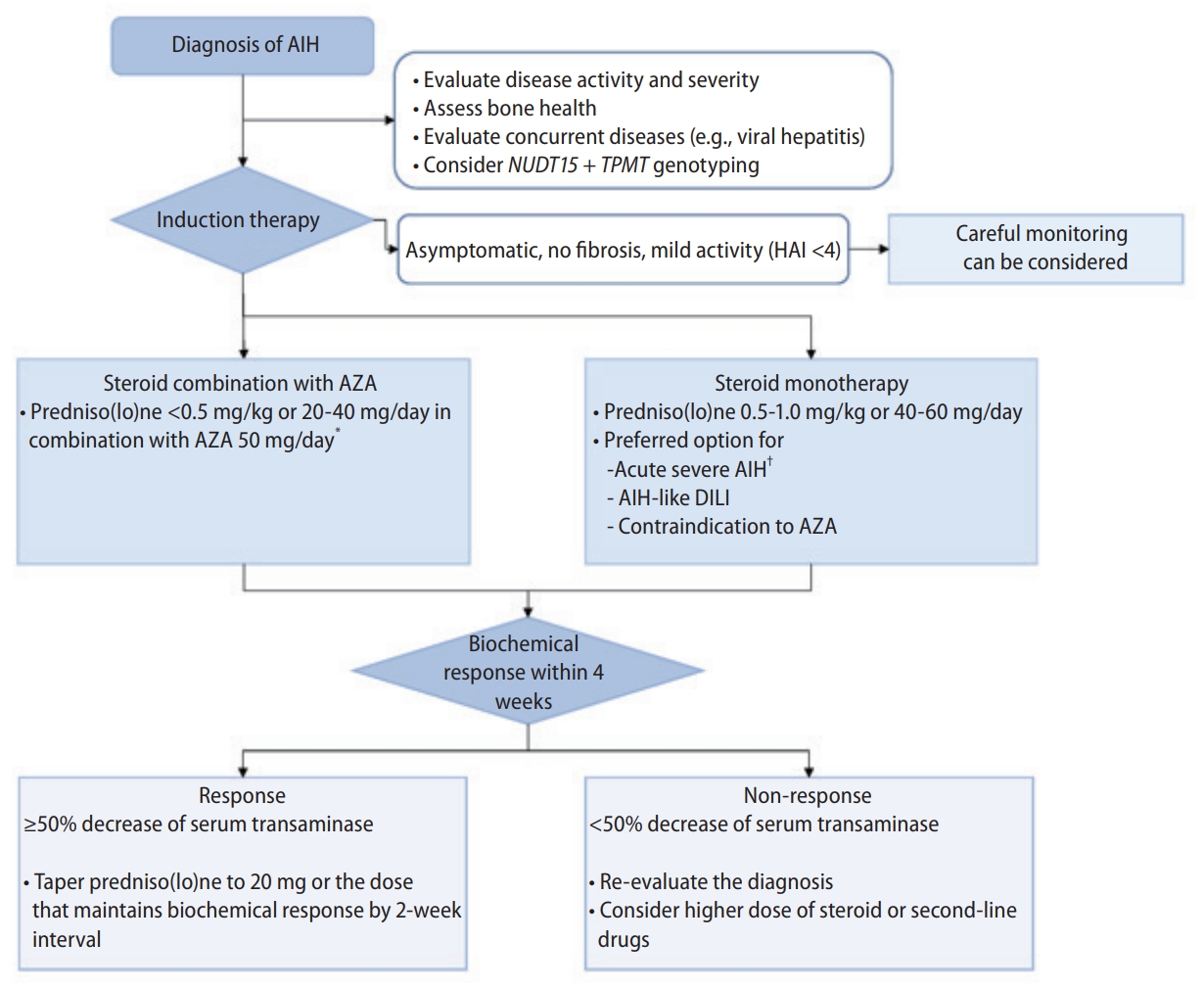
Induction strategy for autoimmune hepatitis. AIH, autoimmune hepatitis; AZA, azathioprine; DILI, drug-induced liver injury; HAI, hepatitis activity index; NUDT15, Nudix hydrolase15; TPMT, thiopurine S-methyltransferase. *Delayed institution of AZA by 2 weeks can be considered. †Emergent evaluation for liver transplantation should be considered for patients with ALF.
The efficacy of prednisolone alone or AZA combination therapy in AIH has been demonstrated through several randomized controlled trials [154-158]. A systematic review of these randomized controlled trials showed similar remission rates between predniso(lo)ne monotherapy and AZA combination therapy (42% vs. 43%; relative risk [RR], 0.98; 95% CI, 0.65–1.47), and fewer drug-related adverse events occurred with AZA combination therapy [163]. Prednisolone and AZA combination therapy is similar in efficacy to prednisolone monotherapy, but has advantages in terms of adverse events and is preferred as the first-line treatment [20]. On the other hand, when the treatment duration is expected to be shorter than 6 months, such as AIH-like DILI, or when AZA is contraindicated, prednisolone monotherapy is recommended [21].
According to a retrospective study on the initial dose of prednisolone, when comparing prednisolone 30 mg and 40 mg as a combination therapy with AZA, the remission rate at 3 months of treatment was higher in the 40 mg group (69.2% vs. 43.8%, P=0.031), but there was no statistically significant difference in remission rates at 6 months and 12 months (79.5% vs. 59.4%, P=0.065; 89.5% vs. 80.6%, P=0.30) and in recurrence rate during maintenance therapy (35.9% vs. 50%, P=0.23) [164]. In a multicenter retrospective study conducted in Europe, there was no significant difference in biochemical response rates at 6 months between the high-dose prednisolone (≥0.5 mg/kg/day) and low-dose prednisolone (<0.5 mg/kg/day) treated groups (70.5% vs. 64.7%, P=0.61). There was no statistically significant difference in glucocorticoid-related adverse events between the two groups, but the incidence of glucocorticoid-induced diabetes (7.7% vs. 3.9%, P=0.13) and osteoporosis (6.4% vs. 2.6%, P=0.09) was higher in the high-dose administration group [165]. A meta-analysis of 25 studies on glucocorticoid doses reported that high-dose glucocorticoid (60 mg/day or 1 mg/kg/day) administration had a higher biochemical remission rate (79% vs. 72%) compared to low-dose glucocorticoids (40–50 mg/day or 0.5 mg/kg/day), but liver transplantation or mortality (3% vs. 1%) and glucocorticoid-related adverse events (42% vs. 39%) were also higher [166].
In combination therapy for inducing remission of AIH, AZA can be administered simultaneously with prednisolone or administered sequentially with an interval of about 2 weeks. When administered sequentially, the response to prednisolone can be evaluated with prednisolone administration alone during the first 2 weeks of treatment, eliminating the uncertainty of diagnosis and accurately evaluating the treatment response by excluding AZA-induced hepatotoxicity that may rarely occur in severe liver disease [20,167]. In addition, the risk of complications can be predicted by evaluating the patient’s NUDT15 (Nudix hydrolase 15) and TPMT (thiopurine S-methyltransferase) gene mutations during the first 2 weeks of not administering AZA [21]. AZA is initially administered in combination with prednisolone at a dose of 50 mg/day and may be increased to 150 mg/day or 2 mg/kg/day depending on toxicity and response to treatment. If a NUDT15 or TPMT gene variant is present, AZA metabolism is impaired and the risk of cytopenia due to bone marrow suppression increases. Since patients homozygous for any risk variants have almost no enzymatic activity, prednisolone monotherapy or alternative therapy without AZA should be considered. Even the patients who are heterozygous for risk variants can also have a risk of bone marrow suppression, so the dose of AZA should be reduced. When considering a dose of 2 mg/kg/day or more, the starting dose of AZA should be reduced by 30–80% and adjusted based on the degree of myelosuppression [168]. In particular, more attention should be paid to patients with risk variants in both the NUDT15 and TPMT genotypes.
If there is a biochemical response with an initial dose of prednisolone and AZA, monitoring should be conducted every 1–2 weeks and prednisolone gradually reduced to a dose that maintains the biochemical response or 20 mg/day while maintaining AZA. Then, monitoring should be performed every 2–4 weeks and prednisolone gradually reduced by 2.5–5 mg to maintain 5–10 mg/day or a dose that maintains the biochemical response. After achieving a complete biochemical response, prednisolone can be discontinued while maintaining AZA. In several randomized controlled trials of maintenance therapy [169-171], AZA alone maintenance therapy showed a higher sustained remission rate than predniso(lo)ne alone maintenance therapy (92% vs. 68%; RR, 1.31; 95% CI, 1.07–1.70), and there was no significant difference in the sustained remission rate compared to predniso(lo)ne plus AZA maintenance therapy (92% vs. 96%; RR, 1.06; 95% CI, 0.94–1.20) [163]. In addition, high-dose AZA monotherapy (2 mg/kg/day) reduced glucocorticoid-induced adverse events and recurrence, showing a high remission persistence rate of 83% for an average of 67 months [170,172]. If leukopenia or thrombocytopenia occurs during AZA treatment, the dose should be reduced or discontinued; especially, if cytopenia does not recover within 1–2 weeks, AZA should be discontinued. Care should be taken in patients with LC due to the high incidence of AZA-induced cytopenia [173,174]. When AZA administration is impossible due to adverse events, the lowest dose of prednisolone alone can be administered as maintenance therapy. However, long-term administration of prednisolone in doses exceeding 10 mg per day may cause frequent steroid-related adverse events. Therefore, it is recommended to administer the lowest dose of prednisolone that maintains the biochemical reaction, keeping the dose below 10 mg/day if possible [175].
Alternative first-line therapy
According to a prospective randomized controlled study on the efficacy and safety of budesonide and AZA combination therapy, budesonide (9 mg/day) and AZA (1–2 mg/kg/day) combination therapy showed a significantly higher 6-month biochemical remission rate (60% vs. 38.8%, P=0.001) and lower side effects of glucocorticoids (26% vs. 51.5%, P<0.001) compared to prednisolone (40 mg/day) and AZA (1–2 mg/kg/day) combination therapy in patients with AIH without cirrhosis [176]. Since budesonide has a high (>90%) first-pass effect in the liver, it has fewer adverse effects caused by glucocorticoids and can be advantageous in terms of bone density in the long term [177-179]. However, budesonide bypasses the liver due to portal systemic shunt in patients with LC, which reduces the efficacy of glucocorticoids and increases glucocorticoid-induced adverse effects [180,181]. Therefore, as a first-line treatment, budesonide and AZA combination therapy can be selectively considered in patients with AIH without cirrhosis who are highly likely to have glucocorticoid-induced adverse effects, or when prednisolone administration is not possible due to adverse effects. However, as of 2022, oral budesonide cannot be used in South Korea as it is not commercially available.
If the administration of AZA is not possible, mycophenolate mofetil (MMF) can be used as an alternative first-line treatment. A single-center prospective study reported a remission rate of 71.6% when MMF (1.5–2 g/day) was administered in combination with prednisolone as the first-line treatment for AIH, and 78.2% of them maintained remission with MMF-only (1–1.5 g/day) maintenance therapy [182]. As a result of a meta-analysis of seven prospective and retrospective studies, the predniso(lo)ne-MMF combination therapy showed significantly higher normalization rates of AST, ALT (OR, 1.49; 95% CI, 1.02–2.18), and IgG (OR, 1.87; 95% CI, 1.21–2.88), and significantly lower non-response rates (OR, 0.55; 95% CI, 0.36–0.85) compared to the predniso(lo)ne-AZA combination therapy [183]. Although MMF has been proven to be effective when administered in combination with prednisolone as a first-line treatment for AIH, it is recommended as an alternative treatment to AZA due to insufficient studies to date.
Acute severe AIH and acute liver failure due to AIH
In acute severe AIH or ALF due to AIH, the efficacy and optimal dose of glucocorticoid treatment have not yet been clearly proven due to the high rate of treatment failure [184], the possibility of delayed liver transplantation due to initial medical treatment [185,186], and the risk of infection due to glucocorticoid administration [187].
According to a study of 72 patients with acute severe AIH with jaundice, the treatment failure rate was as high as 18% when treated with predniso(lo)ne at a dose of 40 to 60 mg/day. The higher the serum level of bilirubin and PT, the higher the risk of treatment failure, and the higher the risk of death and emergency liver transplantation in case of treatment failure [184]. In a French single-center retrospective study of 16 patients with acute severe AIH or ALF due to AIH (63% of whom had hepatic encephalopathy, median PT INR of 5.4), 12 patients received corticosteroid therapy, 11 patients underwent liver transplantation, one patient died, and three pateints developed severe sepsis, reporting that corticosteroid treatment increased the incidence of infection without improving the prognosis [187]. Meanwhile, in a study of 32 patients with acute severe AIH without hepatic encephalopathy, all of the untreated patients required emergency liver transplantation, whereas only 43% of patients treated with corticosteroids (mean dose of 40 mg/day of predniso(lo)ne) received emergency liver transplantation (P=0.004), and the sepsis incidence and mortality were not significantly different between the two groups (11% vs. 26%, P=0.6; 22% vs. 17%, P=0.99) [188]. Another study in patients with acute severe AIH also reported favorable long-term survival (97% during 5.3 years) without progression to liver failure or liver transplantation with early administration of high-dose glucocorticoids (1.5 mg/kg/day of prednisolone) [189].
Collectively, glucocorticoid treatment (0.5–1 mg/kg/day of predniso(lo)ne alone) in patients with acute severe AIH was effective and did not significantly increase the risk of infection[185,186,188,189]. Glucocorticoids treatment may be considered in patients with ALF due to AIH, being cautious of complications, such as infection [187]; however, in particular, patients with a Model for End-stage Liver disease (MELD) score >40 had lower overall survival with glucocorticoids treatment [190]. When treating acute severe AIH with glucocorticoids, it is important to promptly evaluate the clinical course and treatment response within 1 to 2 weeks and proceed with liver transplantation if there is little effect. Liver transplantation should be considered without delay if liver enzyme levels do not decrease, clinical symptoms worsen, or hepatic encephalopathy progresses [184,186,191]. Accompanying hepatic encephalopathy means ALF, and in this case, liver transplantation is more helpful in prognosis than glucocorticoids treatment [184,187].
[Recommendations]
1. Prednisolone plus AZA (A1) or prednisolone alone (A2) is recommended as the first-line treatment for AIH.
2. After achieving a complete biochemical response in patients with AIH, AZA alone or prednisolone at the lowest dose capable of maintaining remission plus AZA is recommended as the maintenance treatment. (A1)
3. Prednisolone alone (0.5–1 mg/kg/day) can be administered in patients with acute severe AIH (C2), but liver transplantation is considered when there is no response to treatment or when liver failure accompanied by hepatic encephalopathy occurs. (C1)
Treatment withdrawal
Clinical parameters of treatment withdrawal
The goal of AIH treatment is to reduce mortality and increase survival by continuously controlling disease activity. However, it is practically difficult to determine the end of immunosuppressive therapy after the achievement of treatment goals. Therefore, alternative clinical parameters that are readily measurable and reflect the achievement of treatment goals are needed when considering the withdrawal of treatment. In clinical practice, complete normalization of serum transaminases and IgG levels for at least 2 years have been proposed as requirements before attempting treatment withdrawal [18,21,192]. Histologic examination prior to treatment withdrawal has been the preferred strategy, as histologic features are predictors of fibrosis progression and relapse. However, in adult patients with and without treatment withdrawal liver biopsy guidance, the rates of relapse were similar in both groups (30% vs. 21%) after treatment for at least 2 years following complete biochemical remission [193]. Of 28 patients in biochemical remission for at least 2 years before treatment withdrawal, 15 (54%) patients remained in biochemical remission after withdrawal, and five of 11 (46%) patients who showed histologic remission subsequently relapsed during a median follow-up of 28 months [192]. Since liver biopsy is not always available in clinical practice, it may not be mandatory before treatment withdrawal in all adult patients [20,21]. However, for patients with poor adherence to treatment or severe clinical symptoms, a liver biopsy should be considered prior to treatment withdrawal [18].
Non-invasive assessment of fibrosis by transient elastography might aid in the decision of treatment withdrawal. In a recent study, liver stiffness decreased by 7.5% per year in patients with biochemical remission, whereas those who were not in biochemical remission showed an increase in liver stiffness by 1.7% per year [135]. The average liver stiffness measurement of pre- and post-treatment was 8.2±6.7 kPa and 6.4±3.2 kPa in the remission group, and 8.1±5.8 kPa and 9.2±9.1 kPa in the non-remission group, respectively. However, a cutoff of liver stiffness for predicting remission or the association between liver stiffness and long-term outcome after treatment has not been determined yet. Therefore, its role in predicting relapse after withdrawal is unknown, and further studies are required.
Follow-up after treatment withdrawal and treatment of relapse
Relapses are very common after treatment withdrawal in AIH patients. Even patients with complete biochemical response for ≥2 years have shown relapse rates of 20–46% [192,194]. Most relapses occur within 6 to 12 months. About 50% of patients develop relapse within the first 3 months, and the frequency decreases after the first year to about 3% per year [195]. However, since late relapse also can occur, patients should be closely monitored in 3- to 6-month intervals for the first 1 year after treatment withdrawal and in 6-to 12-month intervals thereafter [20,21].
Relapse is usually asymptomatic, manifested by mild elevation of serum transaminases [196]. However, delayed or failed detection resulted in fibrosis progression in 10% of cases, and deterioration of hepatic function in 3% [197]. Therefore, early detection and prompt treatment of relapse is required. A liver biopsy is usually not mandatory to confirm the relapse, as the elevation of serum ALT is highly predictive. Various factors have been proposed as factors associated with relapse, such as slow response to treatment and short treatment duration [192,193], psychological stress or concomitant autoimmune diseases [198,199], AST or ALT levels above ULN or high IgG levels (>1.5 g/dL) at discontinuation of treatment [146,152,192,200], infiltration of plasma cells in the portal area at discontinuation [201], and prednisolone monotherapy [169], while the presence of clinical or histological cirrhosis at initial diagnosis or treatment withdrawal was not associated with relapse [194,197,199,202].
Treatment of the relapse aims to resume the initial treatment which led to remission [196,199,202-204]. Treatment should be initiated at induction doses, followed by glucocorticoid tapering. Once biochemical remission is re-established, the dose of AZA can be increased to 2 mg/kg daily, as glucocorticoid is reduced to the lowest dose needed to maintain remission or fully withdrawn. [204,205]. Patients intolerant of AZA can be treated with MMF or the lowest dose of glucocorticoid monotherapy needed to maintain biochemical remission [204,206].
[Recommendations]
1. Treatment withdrawal is considered in patients with AIH showing complete biochemical remission for at least 2 years (C1). A liver biopsy prior to treatment withdrawal may be considered if clinically necessary (C2).
2. Relapse after treatment withdrawal requires prompt reinstitution of the initial induction therapy in patients with AIH (C1). After achievement of complete biochemical response, transition to a long-term maintenance therapy may be considered (C2).
Pretreatment evaluation and monitoring
It is necessary to perform relevant tests to assess and manage treatment-related adverse events before or during the treatment of AIH [20,21].
Bone density assessments
Osteoporosis is associated with fractures which may lower the health-related quality of life. The initial fracture risk assessment or bone mineral density test should be performed before initiating glucocorticoid treatment, since the use of glucocorticoid can cause osteoporosis. The risk of osteoporotic fracture increases in patients receiving more than 7.5 mg of prednisolone daily or a cumulative dose of at least 5 g in the past year [207].
The Korean glucocorticoid-induced osteoporosis guideline recommends bone mineral density (BMD) testing within 6 months of the initiation of glucocorticoid treatment if patients aged <40 years have a history of osteoporotic fracture or other risk factors for osteoporosis (thyroid disease, history of smoking or alcohol use, etc.). It also recommends assessing the fracture risk using FRAX (Fracture Risk Assessment Tool, https://www.sheffield.ac.uk/FRAX/tool.aspx) and measuring BMD for patients aged ≥40 years within 6 months of the initiation of glucocorticoid treatment [208].
The risk of fracture should be reassessed every year if glucocorticoids are used continuously [208]. FRAX and BMD reassessment should be performed every 1 to 3 years for patients ≥40 years of age who are taking glucocorticoids continuously, but not treated with osteoporosis medications beyond calcium and vitamin D [208]. If patients <40 years of age are taking high dose of glucocorticoids (prednisolone ≥30 mg/day and cumulative dose >5 g/year) or have a history of osteoporotic fracture, Z-score <-3 at hip or spine BMD, or other osteoporosis risk factors, BMD test should be repeated every 2 to 3 years [208].
According to the Korean glucocorticoid-induced osteoporosis guideline, patients are recommended to take calcium (1,000–1,200 mg/day) and vitamin D (800 IU/day), and maintain adequate vitamin D concentrations (≥20 ng/mL) [208].
About 30% of patients with chronic liver disease are known to have osteoporosis regardless of the use of glucocorticoid [209]. The European guideline on nutrition in chronic liver disease recommends repeating BMD evaluation after 2 to 3 years even in patients with normal BMD. Supplementation of calcium (1,000–1,500 mg/day) and vitamin D (400–800 IU/day) is also recommended, although there is insufficient data confirming that these supplements can prevent bone loss in patients with liver disease [209].
Viral hepatitis assessments
Vaccination or infection status of viral hepatitis should be reviewed before initiating immunosuppressive therapy for AIH. If HBV infection status is unclear, screening for hepatitis B surface antigen (HBsAg) and anti-HBc IgG is necessary prior to immunosuppressive therapy. If either HBsAg or anti-HBc IgG is positive, hepatitis B virus (HBV) DNA test should be performed [210].
Vaccination against hepatitis A virus (HAV) and HBV should be given to patients with AIH, since hepatitis A or hepatitis B can increase morbidity and mortality in patients with preexisting chronic liver disease [211]. Accordingly, HAV and HBV vaccination is recommended for patients with AIH [20,21]. HAV vaccination should be given in a 2-dose series at 0 and 6–18 months to patients <40 years of age regardless of the test status for antibody to HAV or seronegative patients ≥40 years of age [212]. If HBsAg and anti-HBs are negative, HBV vaccination of 3-dose series should be administered on a schedule of 0, 1, and 6 months for patients who have not been vaccinated [213].
Out of 15 patients with autoimmune liver diseases (10 patients receiving immunosuppressive therapy), 100% developed anti-HAV after vaccination. For HBV, 16 (eight patients receiving immunosuppressive therapy) out of 21 patients (12 patients receiving immunosuppressive therapy) developed antibody against HBV after vaccination; the response rate (76%) was rather low, considering that the protective antibodies are generally detected in more than 95% after HBV vaccination [214,215]
Genotyping
AZA, one of the thiopurines, can cause adverse effects such as myelosuppression. Pretreatment testing for genotypes associated with drug metabolism may predict the incidence of myelosuppression; TPMT and NUDT15 polymorphisms are well-known for their association, respectively.
TPMT
TPMT is an enzyme that metabolizes AZA, and TPMT deficiency increases 6-thioguanine nucleotides (6-TGN) leading to myelosuppression [20,168]. TPMT activity is associated with TPMT single nucleotide polymorphism. Patients with homozygous TPMT have very low enzyme activity and risk of developing severe myelosuppression; therefore, alternative therapy other than AZA should be considered [20,168].
A previous study reported that the TPMT test did not predict AZA-associated adverse events [173]. The utility of the TPMT test remains uncertain since the dose of AZA used in patients with AIH (50–150 mg/day) is generally lower than that used in patients with inflammatory bowel diseases [174]; however, Western guidelines recommend pretreatment testing for TPMT activity considering the severity of myelosuppression [21]. On the other hand, the frequency of TPMT-deficient allele was less than 5% in Asia [20,216], and a retrospective study of Korean patients with Crohn’s disease found TPMT mutations in 3.8% and 1.2% of patients who experienced leukopenia [217]. The correlation between TPMT genotypes and AZA-induced myelosuppression seems quite low in Asian countries, including South Korea.
NUDT15
NUDT15 is an enzyme that is involved in the metabolism of AZA, and NUDT15 deficiency causes myelosuppression [218]. NUDT15 R139C polymorphism is most commonly reported, and grade 3–4 leukopenia develops within 8 weeks of commencing AZA therapy in 100% of homozygous patients [217].
Early leukopenia (within 8 weeks of commencing AZA treatment) occurred in 25.6% of patients who are heterozygous for NUDT15 R139C and 0.9% of patients with the wild type [217]. A couple of Asian retrospective studies reported the prevalence of NUDT15 R139C homozygote as 1–3%, and that almost all of the patients experienced early leukopenia [218]. The prevalence of NUDT15 R139C heterozygote was about 20%, and early leukopenia developed in 20% of those patients. The wild type was observed in 70–80%, and approximately 3% of patients with the wild type experienced early leukopenia. However, the daily dose of AZA was higher than 50 mg, since most studies were conducted for patients with inflammatory bowel diseases. Several variants other than NUDT15 R139C affecting the enzyme activity have been reported, and the prevalence of the wild type, heterozygote, and homozygote was 69–87%, 12–28%, and 1–3%, respectively, in Asians. However, European patients showed a very low prevalence of heterozygotes and homozygotes at 0.5% and 0%, respectively [219].
In a Chinese retrospective study of 113 patients with AIH, leukopenia was developed in 27.3% (6/22) of patients heterozygous and 100% (3/3) of patients heterozygous for NUDT15 R139C, respectively [220]. On the other hand, TPMT (TPMT*3C rs 114235) homozygotes and heterozygotes were 0% and 1.8%, respectively, and leukopenia did not occur [220].
Another Chinese retrospective study of 149 patients with AIH or AIH-PBC overlap syndrome taking AZA for ≥3 months reported 12 patients (8.1%) who experienced leukopenia (<3,000/mm3). Leukopenia occurred in 37.5% (9/24) of heterozygous and 100% (2/2) of homozygous for NUDT15 R139C patients [221]. On the contrary, TPMT genotype did not show any significant correlation with leukopenia.
A randomized controlled trial of 182 Korean patients with inflammatory bowel diseases compared the incidence of myelosuppression between different treatment strategies based on genotyping prior to thiopurine treatment. The intervention group underwent pretreatment genotyping for TPMT, NUDT15, and FMO, and received a low dose of thiopurine (AZA 50 mg/day or 6-mercaptopurine [6-MP] 25 mg/day) or alternative therapy if they carried heterozygotic or homozygotic variants. Patients who did not take pre-treatment genotyping received conventional thiopurine therapy (starting with 50 mg AZA, and then the dose was increased to 2.0–2.5 mg/kg/day by 25 mg every 1–2 weeks). For the first 3 months, the incidence of myelosuppression was significantly lower in the genotyping group at 7.7% compared to the 26.3% in the non-genotyping group (P=0.049) [222]. Another randomized controlled study reported that the incidence of leukopenia was significantly lower in the genotyping-based treatment group compared to the non-genotyping group (23.7% vs. 32.4%, P=0.049) [223].
In Asian countries, including South Korea, NUDT15 variants appear to be more highly correlated with AZA-induced myelosuppression than TPMT variants [224]. Pre-treatment genotyping for NUDT15 and/or TPMT should be considered prior to starting treatment with AZA, especially at a daily dose of >50 mg. Regular monitoring of a complete blood count and blood chemistry is required during AZA treatment.
Other adverse events
Side effects of glucocorticoid include Cushingoid face, weight gain, osteoporosis, diabetes, cataracts, psychosis, and high blood pressure. Cushingoid face and buffalo hump occur in about 50% of patients; diabetes in 15–20%; and hypertension, psychosis, cataracts, and osteoporosis in about 5–10% [155-157,225]. Although adverse effects are observed less frequently after combined treatment with AZA than with glucocorticoid alone, at least 5% of patients experience adverse effects of glucocorticoids [163]. To detect these side effects, periodic physical measurements, blood pressure measurements, and blood tests are required. There is a meta-analysis in which the risk of gastrointestinal bleeding and perforation increases only in hospitalized patients using glucocorticoids [226], but the role of preventive drug administration has not been proven.
The side effects of AZA include nausea, heartburn, hepatotoxicity, hair loss, loss of appetite, fatigue, bruise, bone marrow suppression, increased infection vulnerability, and increased cancer risk. About 25% of patients receiving AZA treatment suffer side effects, and about 10% of patients need to stop the AZA treatment. These side effects are more common in patients with cirrhosis. In about 5% of patients, severe side effects, such as joint pain, fever, skin rash, and pancreatitis, occur within days or weeks of treatment, which results in discontinuation of the drug, and this reaction disappears within several days [20]. Since bone marrow suppression can occur, AZA treatment is not recommended in patients with patients with severe leukopenia (<2.5×109/L), severe thrombocytopenia (<50×109/L), or homozygote mutation in the NUDT15 or TPMT gene. The frequency and intensity of the side effects of AZA depend on the dosage and duration of treatment, the type of combination therapy, and the underlying conditions [227]. A rare case of AZA-induced pneumonia was reported in patients with ulcerative colitis, accompanied by symptoms such as fever, respiratory failure, pulmonary nodular opacity, and ground glass shadowing were observed; and these symptoms disappeared after discontinuation of the drug [228].
If re-use of AZA is inevitable, it can be referred that 64% (9/14) of patients could be re-administered AZA by restarting with dose escalation in a report of patients with inflammatory bowel disease who stopped AZA treatment due to flu-like symptoms, such as severe myalgia, headache, and fever [229].
[Recommendations]
1. For AIH patients ≥40 years of age or patients <40 years of age with high risk factors for osteoporosis, the risk of fracture or bone mineral density should be evaluated before or within 6 months of the initiation of glucocorticoid treatment and followed up at regular intervals depending on the risk of fracture, if glucocorticoid treatment is continued. (C1)
2. Vaccination or infection status of viral hepatitis should be assessed in patients with AIH, and vaccination should be performed if anti-HAV or HBsAg/anti-HBs are negative. (B1)
3. In patients with AIH, complete blood count should be monitored during AZA treatment. (B1) Genotyping for NUDT15 (B2) and/or TPMT (C2) may be considered before initiating AZA treatment.
Second-line treatments
Second-line treatments in AIH are considered in cases of intolerance to treatment, treatment failure, and non-response during initial treatment using glucocorticoid and AZA (Fig. 6). There is insufficient evidence on the standard second-line treatments of AIH, as related studies are few and most were conducted in a retrospective manner with a small population. Additionally, the second-line treatments may differ among intolerance to treatment, treatment failure, and non-response. A systematic review indicated that the proportions of intolerance to treatment, treatment failure, and non-response during the first-line treatments were 13%, 14%, and 7%, respectively [230]. Therefore, the diagnosis of AIH should be reconfirmed and medication adherence should be re-evaluated in patients with non-response to the first-line treatments.

Maintenance strategy for patients with autoimmune hepatitis showing biochemical response to prednisolone and/or azathioprine induction therapy. 6-MP, 6-mercaptopurine; 6-TG, 6-thioguanine; AZA, azathioprine; MMF, mycophenolate mofetil.
Second-line agents
Mycophenolate mofetil (MMF): MMF is an inosine-5’-monophosphate that decreases both T-cell and B-cell proliferations, and it suppresses antibody formation and cell-mediated immunity [231]. MMF (1–2 g/day) is given with glucocorticoids for patients intolerant of AZA or who have incomplete or no response to first-line treatment. An Australian multicenter retrospective study demonstrated that 3-month biochemical response was 61.0% (57.1% in treatment intolerance and 61.9% in suboptimal response) in 105 patients who received MMF after first-line treatment (42 patients with drug intolerance and 63 patients with incomplete or no response to standard therapy) [232]. A meta-analysis showed that the biochemical response rate was 58.0–59.6% in patients who received MMF as a second-line treatment for AIH (73.5–82.0% in drug intolerance and 32.0-40.8% in incomplete or no response to standard therapy) [233,234].
Adverse events of MMF include nausea, vomiting, diarrhea, heartburn, headache, dizziness, skin rash, and infection. MMF is contraindicated in pregnant patients due to its teratogenic effect [235]. Adverse events are observed in 14.0–24.1% of patients receiving MMF. The most common adverse event is leukopenia, which can be controlled by dosage reduction, followed by gastrointestinal symptoms such as nausea, vomiting, and diarrhea. Rarely, severe leukopenia, sepsis, myalgia, pancreatitis, headache, hair loss, and paresthesia may also develop [233,234].
Cyclosporine: Cyclosporine, a calcineurin inhibitor, can be given in combination with glucocorticoids as a second-line treatment for AIH (2–3 mg/kg/day). The biochemical response of cyclosporine was 80% in five patients who showed incomplete or no response to glucocorticoid and AZA therapy [236]. Adverse advents, such as nephrotoxicity and changes in facial appearance (hypertrichosis), are commonly observed. However, evidence is lacking to support the use of cyclosporine as a second-line treatment.
Tacrolimus: Tacrolimus is a more potent calcineurin inhibitor that has a lesser change in facial appearance than cyclosporine. Tacrolimus is used in combination with glucocorticoids as a second-line treatment (0.5–6 mg/day). A European retrospective study showed that a biochemical response with tacrolimus as a second-line treatment was 52.9% in 17 AIH patients with intolerance to AZA treatment (n=1) and incomplete response to standard treatment (n=16) [237]. A meta-analysis revealed that the biochemical response rate was 68.9% in AIH patients who received tacrolimus as a second-line treatment (56.6% with intolerance and 59.1% with incomplete or no response to standard therapy) [234]. Adverse events of tacrolimus include neurologic symptoms, gastrointestinal symptoms, nephrotoxicity, diabetes mellitus, hypertension, and hair loss [238]. Nephrotoxicity can occur frequently in patients with high blood levels of tacrolimus. Therapeutic drug monitoring of tacrolimus is necessary since between-individual variability, drug-drug interactions, and drug-food interactions may affect the blood tacrolimus levels [239]. Adverse events of tacrolimus were reported in 25.5% [234].
Few studies compared MMF and tacrolimus as second-line treatments for AIH. A global retrospective study analyzed a total of 201 patients receiving MMF (n=121) or tacrolimus (n=80) as a second-line treatment for AIH, which consisted of 108 patients with intolerance and 93 patients with incomplete or no response to first-line treatment [240]. No significant differences were observed in the complete response rate between the two groups (MMF, 69.4% vs. tacrolimus, 72.5%; P=0.639). However, the complete response rate to tacrolimus (56.5%) was higher than that to MMF (34.0%) in patients who showed incomplete or no response to the standard therapy (P=0.029). Adverse events and liver-related mortality were not significantly different between the two groups [240]. However, it needs to be validated with prospective studies since the study might include several biases caused by the retrospective manner. The practice guideline for AIH released by the American Association for the Study of Liver Diseases (AASLD) in 2019 performed a meta-analysis on the comparison between MMF and tacrolimus as the second-line treatments for AIH. Although the meta-analysis revealed that both of the two drugs were effective in AIH as the second-line treatments, the AASLD guideline preferred MMF to tacrolimus considering the drug accessibility and safety profile [21]. However, further studies are needed to clarify due to a lack of high-quality evidence.
6-Mercaptopurine, 6-thioguanine, allopurinol: 6-MP can be administered in patients with intolerance to AZA for AIH. 6-TGN, an active metabolite, is derived from 6-MP, which is the first metabolite of AZA. Although 6-MP is not more effective than AZA, the use of 6-MP can improve medication adherence if there is intolerance to AZA (25–50 mg/day) [241]. In a study in which AZA was changed to 6-MP as a second-line treatment for a total of 22 patients after combination therapy of glucocorticoid and AZA, 75% of 20 patients with AZA intolerance showed biochemical remission, but two patients who had an inadequate response to AZA treatment did not respond [242]. Adverse events of 6-MP include leukopenia, headache, nausea, vomiting, diarrhea, oral ulcer, skin rash, and arthralgia.
6-Thioguanine (6-TG) is metabolized into 6-TGN, active metabolite of AZA. 6-TG can be given to patients with intolerance to AZA for AIH (20 mg/day). A biochemical response with 6-TG as a second-line treatment was 58% in 49 Dutch patients who had intolerance or insufficient response to AZA or 6-MP [243]. Adverse events of 6-TG include nausea, vomiting, poor oral intake, oral ulcer, and headache. 6-TG should be used with caution because of its hepatotoxicity. A meta-analysis showed that hepatic sinusoidal obstruction syndrome developed in 9–25% of 4,849 patients who received 6-TG for inflammatory bowel disease or acute lymphocytic leukemia. The risk of hepatic sinusoidal obstruction syndrome increases with the above daily dosage of 6-TG 25 mg [244].
Allopurinol (100 mg/day) can be added to AZA and glucocorticoid in AIH patients with intolerance to AZA or non-response. The immunosuppressive effect of AZA decreases if it is not metabolized to 6-TGN, active metabolite, but to 6-thiouric acid (6-TU) through xanthine oxidase [235]. Allopurinol can be used in AIH by inhibiting xanthine oxidase that induces AZA to 6-TGN. Low dosage of AZA with allopurinol may be one of treatment options as the second-line treatment. Although, studies on the use of allopurinol for AIH are insufficient, a study reported that the biochemical response rate of adding allopurinol in eight patients who failed with AZA was 88% [245].
Sirolimus, everolimus: Sirolimus and everolimus, mammalian target of rapamycin (mTOR) inhibitors, block IL-2-mediated signal transduction. Subsequently, this decreases the response of T-cell activation by cytokines and the formation of antibody, which prevents cell-cycle progression and proliferation [246]. Further studies are warranted to clarify the use of sirolimus or everolimus as the second-line treatments for AIH, as previous studies have been conducted only for a small number of patients [247,248].
Infliximab, rituximab: Infliximab, a tumor necrosis factor (TNF)-alpha inhibitor, has been tried as a second-line treatment. Of 11 patients receiving infliximab for non-response to treatments, including AZA for AIH, AST/ALT and serum IgG levels were normalized in eight patients and six patients, respectively. However, infection-related complications were observed in seven patients [249]. TNF-alpha antagonists, including infliximab, should be used with caution as they can increase the risk of DILI, including AIH-like DILI [250,251].
Rituximab is a monoclonal antibody against CD20, a surface antigen of B lymphocyte. Rituximab is firstly introduced to treat B-cell lymphoma and has been tried to treat a variety of autoimmune diseases. A phase 1 clinical trial on the use of rituximab was conducted for six AIH patients with treatment failure to glucocorticoid and AZA. After the administration of rituximab 1,000 mg with two sessions at 2-week intervals, all of the patients had decreased levels of AST, ALT, and serum IgG, and then a reduction in the glucocorticoid dosage. Serious adverse events were not observed [252]. However, further studies on rituximab for AIH are warranted.
[Recommendations]
1. In AIH patients who have failed first-line treatments, the confirmation of the diagnosis of AIH and medication adherence should be re-evaluated. Then, second-line treatments should be considered in cases with intolerance to treatment, non-response, and insufficient response. (C1)
2. MMF or tacrolimus is preferentially considered as the second-line treatment (C1), and cyclosporine, 6-MP, and 6-TG can also be used in patients with AIH. (C2)
Treatment of AIH in children
Treatment indications
AIH should be suspected and tested if liver disease is suspected in children without other infectious or metabolic causes. If AIH is diagnosed, treatment should be initiated immediately, as it is important to prevent the progression of the disease [32]. In most cases of AIH, except for those with liver failure and hepatic encephalopathy, the remission rate of immunosuppressant treatment is as high as 90%, regardless of the degree of liver damage [37,41,145,253]. Cirrhosis is observed in 40–88% of pediatric patients with AIH at the time of diagnosis [41,254,255]; however, most pediatric patients receive long-term treatment since the mortality rate is low at this age.
Treatment aims
The goals of AIH treatment are to reduce or eliminate liver inflammation, induce complete biochemical response, improve symptoms, and increase life expectancy in the long term [32]. To achieve these goals, most of the patients need continuous maintenance treatment, and a few patients can maintain remission after treatment withdrawal [20]. The ideal complete biochemical response after treatment is the normalization of serum AST, ALT, and IgG [21,144,145]. However, in children, the criterion that negative conversion or low titer of autoantibodies (ANA & SMA <1:20, anti-LKM1 & anti-LC1 <1:10) and histological loss of inflammation should also be considered [32,256]. This is because histological response lags behind biochemical response, and clinical or biochemical remission does not necessarily reflect the histological loss of inflammation [256].
First-line treatments
The traditional first-line treatment for pediatric AIH is the combination of prednisolone and AZA (Fig. 7) [20,21,32,154-157,163,257-260]. Prednisolone should be started at 1–2 mg/kg/day (maximum 60 mg/day) and then tapered over 4 to 6 weeks, and maintained at a dose of 2.5–5 mg/day as aminotransferase levels decrease. Although the timing of AZA initiation is controversial, it is recommended to add prednisolone around 2 weeks after treatment to accurately evaluate the treatment response to prednisolone and exclude AZA-induced hepatotoxicity [21]. AZA is started at the dose of 0.5 mg/kg/day, maintained at 1–2 mg/kg/day, and can be increased to 2–2.5 mg/kg/day until a complete biochemical response is achieved in the absence of toxicity [261]. AZA should be withheld until liver function improves, and prednisolone alone should be given in patients with decompensated cirrhosis or liver failure since AZA may cause hepatotoxicity [21].
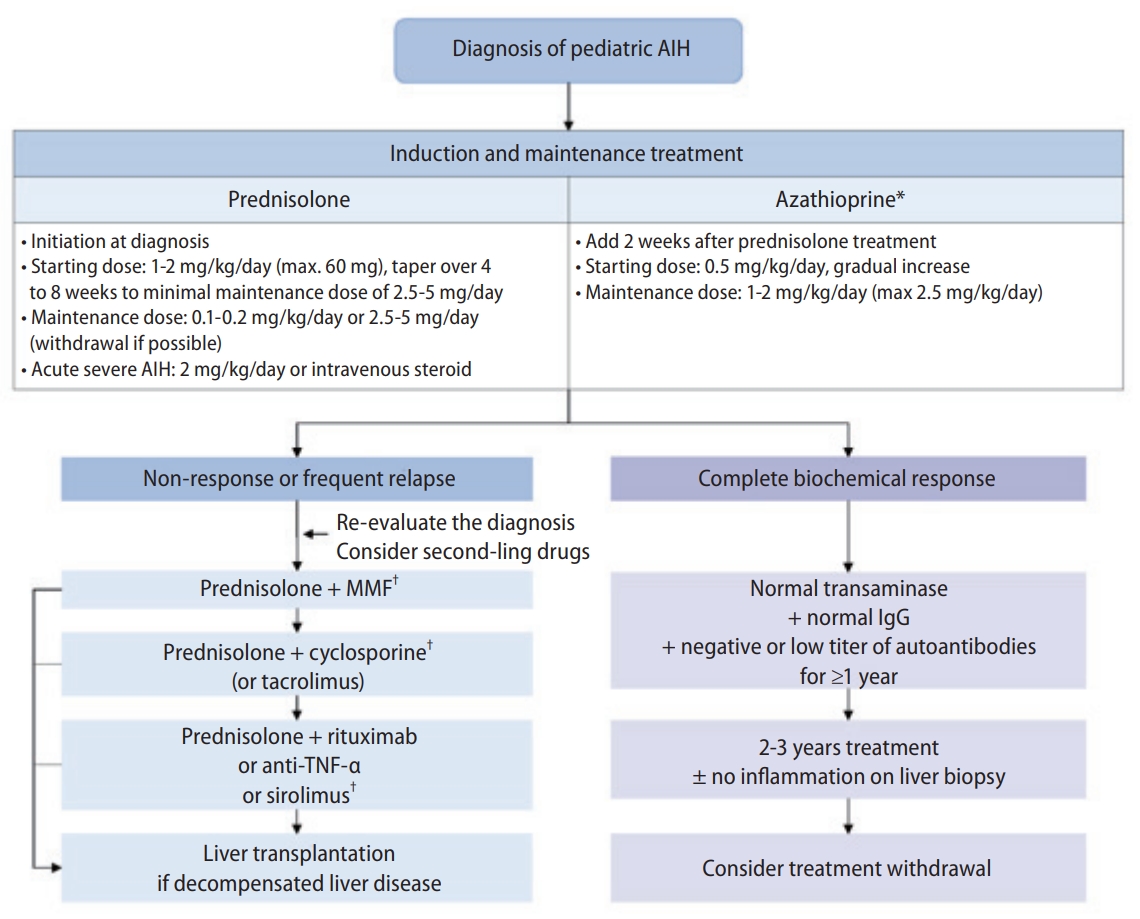
Treatment algorithm for pediatric autoimmune hepatitis. AIH, autoimmune hepatitis; MMF, mycophenolate mofetil; TNF, tumor necrosis factor; IgG, immunoglobulin G. *Do not use in decompensated cirrhosis or liver failure. †Second or third-line treatment should be initiated and monitored only in specialized centers.
Aminotransferase levels usually decrease by 80% or more within 2 months, but it may take several months to fully normalize. In the beginning, liver function tests are performed every 1–2 weeks, and prednisolone can be tapered after achieving a biochemical response. For 4 to 8 weeks after initiation of treatment, the dosage should be adjusted while monitoring biochemical response and drug adverse events every 2 to 4 weeks (Fig. 7).
Children often experience side effects, such as growth failure, weight gain, hypertension, hyperlipidemia, and osteoporosis, as prednisolone is administered for a relatively long period of time [41]. Therefore, prednisolone should be continuously tapered to the lowest dose that can maintain a complete biochemical response or stopped [262,263]. In some pediatric patients, complete biochemical response can be maintained with AZA monotherapy without glucocorticoids [172,261,264,265]. In a study that reduced the prednisolone dose or administered it every other day, the decrease in growth rate improved, but the final height of the patients was shorter than predicted [262]. However, recent studies suggested that appropriate treatment of glucocorticoid in pediatric AIH did not affect the final height. A long-term follow-up study of 28 Egyptian children with AIH reported that the final height was significantly affected by the AIH severity at the onset of the disease, and not by the continuation or the duration of corticosteroid treatment [266]. In another retrospective study of 75 pediatric patients, when low-dose prednisolone (2.5 mg under 12 years old, 5 mg over 12 years old) was prescribed and followed up for about 11 years, the patients’ Z-scores (height, weight, and body mass index) were consistently maintained within the normal range [267]. A subgroup analysis of a randomized controlled trial reported that the remission rate was similar between pediatric patients taking budesonide or prednisone as an induction therapy; however, the weight gain was lower in the budesonide group [268]. Therefore, budesonide can be considered, but oral budesonide is not currently available in South Korea.
The measurement of TPMT activity before starting AZA treatment is recommended in Western guidelines since it may prevent some adverse events [21,32]. However, mutations for NUDT15 rather than TPMT should be considered in Asia [224]. Since most studies were conducted in patients with inflammatory bowel disease, further research is needed for pediatric patients with autoimmune diseases [269].
Second-line treatments
Second-line treatments of AIH can be attempted when patients are intolerant of or do not respond to standard therapy. Drugs used as second-line treatments in children are listed below.
Budesonide: In a double-blind, randomized controlled study of pediatric patients with AIH, budesonide was effective in inducing remission and had fewer side effects than prednisolone [268]. Since more than 90% of budesonide is first transferred to the liver and metabolized, it has few systemic side effects and is also called the local treatment of the liver [270]. However, it cannot be used in patients with LC or poor liver function, and it is not marketed as an oral medication in South Korea.
Mycophenolate mofetil (MMF): In a retrospective study, MMF was used in combination with corticosteroids at doses of 20–40 mg/kg twice daily in 26 children with AIH who did not respond to the first-line treatment. Among them, 18 children responded to MMF, and AST normalized in 14 patients. Six of the eight patients who did not respond to MMF had autoimmune sclerosing cholangitis [271]. The most common adverse event was leukopenia, but there were no serious side effects. In a single-center prospective observational study including two 16- and 17-year-old children, all two children showed normalization of ALT when using MMF [272]. However, the second-line treatment of MMF still remains controversial due to some studies demonstrating the poor effect of MMF, especially in children who do not respond to AZA [37,273-275].
According to a meta-analysis of second-line treatments in pediatric AIH patients who did not respond to the standard therapy, the response rate was the highest with cyclosporine; however, the adverse event rate was also the highest. MMF showed the second-highest response rate and a low adverse event rate [276]. Calcineurin inhibitors can be considered as an alternative option if there are persistent adverse events to MMF (headache, diarrhea, nausea, hair loss, leukopenia, etc.) [32,276].
Cyclosporine: In a Canadian study, treatment-naïve pediatric AIH patients were treated with cyclosporine (4 mg/kg/day in 3 divided dose) alone for 6 months, and then maintained with prednisolone and AZA. Among them, 72% of patients showed normalization of ALT level and improvement in growth rate within 6 months of treatment, with few side effects [277,278]. According to these results, cyclosporine is used as a first-line treatment for AIH in children in some regions, but the level of evidence is still weak [32]. A retrospective study was performed to evaluate the effect of cyclosporine for 13 pediatric patients with type 2 AIH. The patients were treated with cyclosporine due to intolerance (n=8) and non-response (n=5) to the first-line treatment. When cyclosporine was used at a blood concentration of 200–250 ng/mL at the beginning of treatment and maintained at a blood concentration of 100–150 ng/mL for 1 year after remission, the ALT levels were normalized within 6 months in both groups [279]. Additional studies on successful maintenance therapy using cyclosporine in children and adolescent patients were reported [280,281]. In a randomized trial comparing the combination therapy of prednisolone and AZA to cyclosporine in 26 pediatric patients, the treatment effect and safety of the combination therapy and cyclosporine were similar [282].
Tacrolimus: Tacrolimus is a calcineurin inhibitor that is more effective and has fewer side effects than cyclosporine, a drug with the same mechanism. Although the use of tacrolimus has not been studied much in pediatric AIH patients, a non-randomized prospective study revealed that tacrolimus showed an effect of reducing the dose of prednisolone and AZA when used at a target blood concentration of 2.5–5 ng/mL in 17 pediatric patients. However, tacrolimus monotherapy did not induce a complete biochemical response, and adverse events such as headache, abdominal pain, chronic inflammatory bowel disease, and deterioration of liver function occurred [283]. A multinational retrospective study compared the effects of MMF and tacrolimus in 38 pediatric patients who were intolerant to corticosteroids or AZA and non-responsive to the first-line treatment [284]. Complete biochemical response was seen in 88.9% of MMF and 87.5% of tacrolimus in the intolerant group. In the non-response group, a complete response rate was 22.5% in MMF and 50% in tacrolimus. There was no statistical significance between the two drugs [284].
Rituximab, infliximab, sirolimus: Rituximab is an anti-B lymphocyte monoclonal antibody. According to a case report on children with AIH who do not respond to first-line treatment, MMF, and cyclosporine, there was a decrease in liver enzyme levels and IgG was maintained for 12 to 26 months after rituximab treatment (every 4 weeks to 6 months intravenously). No serious side effects were observed [285].
Infliximab may be attempted as a rescue treatment, as some case reports have shown its effect on refractory pediatric AIH [286,287]. However, it has been reported that immune-related hepatitis occurred after infliximab treatment in some pediatric patients with inflammatory bowel disease, and the role of TNF-a in the development of AIH is not fully known. Therefore, infliximab should be used with caution for refractory AIH [288].
Sirolimus is a drug that selectively expands regulatory T cells. In a case report of four pediatric non-responsive patients with AIH, sirolimus was treated and two patients responded to sirolimus. A small series in adult patients with refractory AIH also showed similar results; however, the evidence for sirolimus is insufficient to date [247,289].
Treatment withdrawal
Complete biochemical response, including the normalization of AST, ALT, IgG levels, and negativity or low titer of autoantibodies (ANA & SMA <1:20, anti-LKM1 & anti-LC1 <1:10) should be maintained for at least 2–3 years before treatment withdrawal is tried in children with AIH [32]. Although liver biopsy before the treatment withdrawal is not essential in adults, it is still recommended in children [21,32,41,290].
Most of the studies on treatment withdrawal were conducted retrospectively. In a recent prospective study including adults and children, the treatment was terminated when patients showed normal ALT and IgG levels for at least 2 years in addition to histological remission. The remission maintenance after treatment withdrawal was seen in 67% during the 62-month observation period, which was higher than what was observed in previous studies. However, the number of patients in this study was only 12, and all of them were type 1 AIH patients known to have a relatively low recurrence [291]. Other retrospective studies, including children, reported that histologic findings did not predict recurrence [292].
In a prospective study observing children with AIH for 16 years, treatment withdrawal was attempted in seven children with AIH who showed normal liver enzyme levels for at least 1 year and had no inflammation in follow-up liver biopsy. Among them, three patients were able to stop treatment completely, and three patients with type 1 AIH could discontinue prednisolone and reduce the AZA dose. However, one patient with type 2 AIH recurred 8 months after the treatment withdrawal [293]. In a pediatric study conducted in 1984, treatment withdrawal was attempted after liver biopsy in nine patients. Among them, three patients with type 1 AIH and five patients with type 2 AIH relapsed. In eight patients who relapsed, three patients showed remaining histological inflammation, but five patients confirmed histological loss of inflammation before the withdrawal [262]. Since recurrence of pediatric AIH is relatively common, it is important to prevent recurrence by carefully attempting to withdraw treatment after confirming the complete disappearance of inflammation in liver histology as well as a complete biochemical response.
[Recommendations]
1. Combination therapy of prednisolone and AZA is recommended as the first-line treatment for pediatric patients with AIH. (B1)
2. After achieving a complete biochemical response, pediatric patients with AIH should be treated with AZA monotherapy or combination therapy of prednisolone at the lowest dose that can maintain remission and AZA. (B1)
3. MMF (C1), cyclosporine (B2), or tacrolimus (C2) can be used as second-line treatments in pediatric patients with AIH who show no or incomplete response or intolerance to the first-line treatment.
4. Treatment withdrawal is considered if a complete biochemical response is maintained for at least 2–3 years (C1), and a liver biopsy can be performed before withdrawal (C2) in pediatric patients with AIH.
Treatment of special patient populations
Pregnancy
Pregnancy induces changes in the immune system, which can affect the course of AIH [294]. Pregnancy requires maternal immune tolerance to paternal alloantigens expressed in fetal tissues [295-297]. In general, the activity of autoimmune diseases decreases during pregnancy, and after childbirth, the activity of autoimmune diseases increases, thereby increasing the risk of exacerbation of autoimmune diseases [297,298]. According to studies reporting the clinical course during pregnancy in patients with AIH, serum ALT levels usually decreases during pregnancy, and spontaneous remission during pregnancy is reported in some cases. The risk of acute exacerbation is high during the first 3 months after childbirth [299-302]. However, the course of AIH during pregnancy is very diverse. Acute flares and emergent liver transplantation during pregnancy have been reported [299,300,303]. In particular, the risk of acute exacerbation was high in cases of poor drug compliance, such as discontinuing the drug or reducing the dose against medical advice before or during pregnancy [304]. Therefore, if a patient with AIH is considering pregnancy or becomes pregnant, it is necessary to carefully check for drug compliance, be aware of the possibility of acute exacerbation, and closely conduct follow-ups before, during, and after pregnancy. Not receiving immunosuppressive treatment during pregnancy and having a short remission period (less than 1 year) before pregnancy have been reported as risk factors for acute exacerbations during pregnancy and after childbirth [299,304,305].
AIH and treatment for AIH can affect pregnancy outcomes [294]. There was no difference in the live birth rate between the general population and mothers with AIH [7,306]. However, obstetric complications in patients with AIH were higher than that in the general population [307,308]. According to the analysis of 18,595,345 cases of maternal discharge data from 2012 to 2016 in the United States, the risk of postpartum hemorrhage and maternal death was the same for mothers with AIH compared to the general population, but the risks of gestational diabetes (OR, 2.2; 95% CI, 1.5–3.9), pre-eclampsia and eclampsia (OR, 2.4; 95% CI, 1.6–3.6), and preterm birth (OR, 2.0; 95% CI, 1.2–3.5) were higher for mothers with AIH compared to the general population [307]. In a meta-analysis analyzing the clinical outcome of mothers diagnosed with AIH, the risk of preterm birth was higher than that of the general population (RR, 2.45; 95% CI, 1.66–3.62) [301]. In particular, the risk of preterm birth was high in mothers with portal hypertension or in mothers without biochemical remission before pregnancy [301], and patients with cirrhosis had a higher risk of liver failure or need for a liver transplant [299].
Various drugs used in AIH treatment can affect fetal outcomes [309]. Recent studies have reported no significant association between glucocorticoids and fetal malformations, but glucocorticoids have been reported to increase the risk of cleft lip in the fetus [310,311]. AZA has been reported to be associated with fetal malformations in animal experiments; however, in humans, the association with fetal malformations was not found [312,313]. On the other hand, MMF has been associated with teratogenicity in humans [312]. Therefore, MMF use is contraindicated in pregnancy, and women are recommended to conceive after discontinuing MMF for at least 6 weeks [312]. Glucocorticoids and AZA can be used during pregnancy and lactation [309].
A shorter duration of remission before pregnancy increases the risk of acute exacerbation of AIH during pregnancy [299,305]. Therefore, if possible, it is recommended that pregnancy be considered after the disease has been well-controlled for at least 1 year. For patients planning a pregnancy, the drug contraindicated during pregnancy should be discontinued or changed. If pregnancy is confirmed for a patient receiving glucocorticoids and/or AZA therapy, the drug dose should be minimized to the dose that can maintain remission during pregnancy. During pregnancy, close monitoring for disease activity is required, and special attention is needed especially during the early period after childbirth, as the immunosuppressant requirements to maintain remission can change. During pregnancy, a reduction in immunosuppressant dose can be considered. If immunosuppressants have been reduced during pregnancy, the dose can be preemptively increased after childbirth to the pre-pregnancy dose. The first 3 months after childbirth is a period of high risk for acute exacerbations [299,302], and careful follow-up at short intervals is necessary. The use of glucocorticoids and AZA is not contraindicative to breastfeeding.
[Recommendations]
1. The clinical course of AIH during pregnancy is highly variable, and the risk of flares is high in the early postpartum period, requiring close monitoring during pregnancy and the early postpartum period. (C1)
2. For patients with AIH who plan to become pregnant, family planning should include achieving biochemical remission for at least 1 year prior to conception. (C1)
3. In patients with AIH, MMF is contraindicated, (B1) whereas glucocorticoids and AZA can be maintained during pregnancy. (C1)
Old age
AIH can occur at any age. However, AIH diagnosed in the elderly is characterized by more asymptomatic cases, more often delayed diagnosis, and higher rates of comorbidity, cirrhosis, and extrahepatic diseases, such as thyroid or rheumatic disease [60,314-316]. Elderly patients respond well to the immunosuppressive therapy, with less relapse after treatment withdrawal [317,318]. However, the cut-off age to define the elderly population varies from 60 to 70 years in each study, and some studies have reported no difference in clinical features and treatment responses according to age [315,319].
In elderly patients, drug use to control other comorbidities is common. In these cases, it is sometimes very difficult or impossible to differentiate DILI from AIH [320,321]. DILI and AIH may be differentiated through liver biopsy [322], by monitoring the clinical course after discontinuation of suspected drug, or by monitoring the clinical course after reducing or discontinuing glucocorticoids if glucocorticoids have been used [120].
Elderly patients have a higher rate of comorbidities, such as osteopenia, hypertension, and diabetes, and a higher rate of liver cirrhosis, which can prevent them from receiving immunosuppressive therapy [319]. Immunosuppressive therapy, especially glucocorticoid treatment, can affect the clinical course of comorbidities, such as osteopenia, hypertension, and diabetes. Therefore, when AIH is diagnosed in an elderly patient, comorbidities should be evaluated, and in particular, osteopenia and osteoporosis should be checked in advance [323]. Careful follow-up without immunosuppressive treatment can be considered in elderly patients if AIH is asymptomatic, comorbidity is severe, and there is a high possibility of aggravation of comorbidity after immunosuppressive treatment. However, there is no reason to withhold immunosuppressive treatment just because of age.
AIH overlap syndromes
Overlap syndrome refers to cases that meet the diagnostic criteria for AIH and diagnostic criteria of other autoimmune liver diseases, mainly PBC or PSC. AIH-PBC overlap syndrome has a higher risk of cirrhosis complications than AIH alone or PBC alone [324-326]. In a Korean study, AIH-PBC overlap syndrome showed poorer clinical outcomes compared to AIH alone or PBC alone [50]. AIH-PSC overlap syndrome shows more severe disease and worse prognosis compared to AIH or AIH-PBC overlap syndrome [327].
Clinical presentations and clinical courses are very heterogeneous in patients with AIH [51]; therefore, the treatment of overlap syndrome should empirically focus on predominating features [49]. In a retrospective analysis of a randomized controlled trial evaluating ursodeoxycholic acid (UDCA) response in patients with PBC, 16 patients showed AIH features as well (ALT >5xULN; IgG >2xULN or positive SMA; and moderate to severe lobular inflammation on pretreatment liver biopsy) [328]. In these patients, response to UDCA therapy was similar to those of patients with PBC without features of AIH, suggesting that UDCA therapy without glucocorticoids can be considered for AIH-PBC overlap syndrome with predominant features of PBC [328]. However, the analysis was limited by small size of AIH-PBC patients receiving UDCA therapy. For patients with AIH-PBC overlap syndrome, the combination use of UDCA and glucocorticoid showed biochemical improvement for patients not responding to either UDCA alone or glucocorticoid alone therapy [106]. In a meta-analysis comparing UDCA alone and UDCA with corticosteroids and/or AZA, transplant-free survival was better in the combination treatment group [329]. Hence, UDCA treatment first and sequential addition of immunosuppressive drug can be considered for AIH-PBC overlap syndrome patients if the patient shows predominant features of PBC. Otherwise, combination treatment with UDCA with the immunosuppressive drug is preferred for AIH-PBC overlap syndrome. A previous study reported that immunosuppressive treatment can be helpful in AIH-PSC overlap syndrome, but it was limited by the observational nature of the study [330].
[Recommendations]
1. Combination treatment with immunosuppressive drugs (glucocorticoids and/or AZA) and UDCA is preferred for AIH-PBC overlap syndrome. (B1) If PBC features are predominant, sequential treatment, starting UDCA treatment first and then adding immunosuppressive drug according to the UDCA response, can be considered. (C2)
AIH with viral hepatitis
AIH patients with chronic viral hepatitis B (CHB) have a risk of reactivation of CHB during immunosuppressive therapy, including glucocorticoid. Prophylactic antiviral treatment is recommended if HBsAg or HBV DNA is positive [210]. The high-risk group in which prednisolone is taken ≥20 mg/day for ≥4 weeks and the moderate-risk group in which prednisolone is taken 10-20 mg/day for ≥4 weeks are the indications of prophylactic antiviral treatment. [210,331]. In low-risk cases, in which prednisolone <10 mg/day or antimetabolite such as AZA is taken, it is possible to monitor regularly without antiviral treatment [331]. If the patients of isolated anti-HBc positivity with negative HBsAg and HBV DNA take glucocorticoid or AZA, regular follow-up (HBsAg and HBV DNA every 1–3 months) could be considered. [210].
The aggravation of hepatitis during immunosuppressive therapy is less frequent in chronic viral hepatitis C (CHC) compared with CHB; but rarely, acute exacerbation of CHC may occur during immunosuppressive therapy. Therefore, it is reasonable to treat HCV with direct-acting antivirals before or concomitantly on immunosuppressive treatment. Drug-drug interactions of direct-acting antivirals with immunosuppressant agents should be considered [18].
[Recommendations]
1. Antiviral prophylaxis is recommended if AIH patients have a high or moderate risk of reactivation of CHB during immunosuppressive therapy. (A1)
AIH with non-alcoholic fatty liver disease
Non-alcoholic fatty liver disease (NAFLD) is referred to as a hepatic phenotype of metabolic syndrome, and it often accompanies the factors of metabolic dysfunction such as hypertension, diabetes mellitus, dyslipidemia, and visceral obesity. Since the prevalence of NAFLD is as high as 25% in South Korea, a considerable proportion of AIH patients might have NAFLD in common [332]. In a Japanese retrospective study, 17% of 1,151 patients with AIH had NAFLD. Patients with both AIH and NAFLD showed a lower ratio of female to male, older age, lower elevated ALT level, and lower frequency of glucocorticoid treatment compared to the patients with AIH only [333]. A Western study of 73 patients with AIH reported that 16% and 24% had non-alcoholic steatohepatitis and simple steatosis, respectively [334]. In an interim analysis from an ongoing large-scale multicenter retrospective study in the International Autoimmune Hepatitis Group, 21.6% of 583 AIH patients had NAFLD [335]. A study from the United States presented that the patients with both AIH and NAFLD had 2.5 times liver-related morbidity and 7.6 times higher liver-related mortality compared to the patients with AIH only [334]. Meanwhile, although the positivity of autoimmune antibodies was as high as 23–33% among patients with NAFLD, the proportion of AIH patients confirmed by liver biopsy was less than 2% [336,337]. Therefore, liver biopsy is required to ascertain AIH when autoimmune antibodies are positive in patients with NAFLD.
Treatment for AIH with NAFLD consists of a general approach to NAFLD and specific management for AIH considering NAFLD. A general approach to NAFLD includes weight loss through lifestyle modifications, such as exercise and reduction of intake calorie, and proper medications [338]. Low-dose glucocorticoid and rapid tapering can be specific management for AIH considering NAFLD, but data about this treatment strategy are limited.
Budesonide instead of prednisolone could be used in case of uncontrolled diabetes mellitus without cirrhosis to ameliorate the systemic adverse effects of glucocorticoid, but it is not available in South Korea [176]. Besides that, it can be tried to add AZA or MMF early up to a maximum tolerable dose, and then taper glucocorticoid within 6 months [339].
Recurrent or de novo AIH following liver transplantation
According to data analyzing 4,085 AIH patients registered during 2009–2013 in South Korea, 1.1% of AIH patients received liver transplantation [6,19]. Patients who underwent liver transplantation for AIH had good post-transplantation outcomes, and the 5-year survival and graft survival rates were reported to be 72% and 65%, respectively [340]. After transplantation for AIH, the recurrence rate of AIH was reported to be 10–50% [341], and the recurrence rate of AIH reported by a single institution in South Korea was 14.3% at 5 years [342]. If AIH recurs or de-novo AIH develops after liver transplantation, the clinical course after liver transplantation becomes worse [343]. Studies have shown that the maintenance of glucocorticoids can reduce the risk of AIH recurrence for patients with AIH who received liver transplantation [344]. However, other studies have shown that glucocorticoids can be successfully discontinued in AIH patients after liver transplantation [345]. Strategies for the immunosuppressive drugs in patients undergoing liver transplantation for AIH require further research. In cases of liver transplantation due to AIH, the rates of acute rejection, glucocorticoid resistance rejection, and chronic rejection are higher than those who received liver transplantation for other reasons [346,347] However, it is often difficult to distinguish between rejection and recurrence of AIH, since the criteria for recurrence of AIH in patients after liver transplantation have not yet been established; therefore, care must be taken in interpreting the data [348]. In patients who received liver transplantation for other reasons, AIH can be newly diagnosed during the clinical course, defined as de novo AIH [340,343]. In case of recurrence or new occurrence of AIH, the use of glucocorticoids in addition to calcineurin inhibitors is considered the basis of treatment [341], but optimal immunosuppressant use strategies have not been well-established and require further research.
AIH with hepatocellular carcinoma
In AIH patients diagnosed with HCC, the minimum dose of immunosuppressant that can maintain remission is required, but additional research is needed on the optimal immunosuppressant use strategy. In a study of liver transplant patients, it was reported that calcineurin inhibitors increased the risk of HCC after transplantation, and that the risk of HCC was reduced when mTOR inhibitors were used [349]. Another study demonstrated that MMF reduced the risk of recurrence of HCC after liver transplantation [350]. These results suggest that the risk of HCC development or recurrence may vary depending on the type of immunosuppressive agent. Based on such findings, some studies have suggested that MMF is preferred over AZA as an immunosuppressive treatment for AIH patients with HCC [351]. However, no study has evaluated whether MMF or mTOR-based immunosuppressant treatment can improve the prognosis of AIH patients with HCC compared to treatment with glucocorticoids and AZA.
Immune checkpoint inhibitor-based treatment is recommended for patients with advanced HCC as a first-line option [352]. Immune checkpoint Inhibitors can cause autoimmune-related adverse events [353]. In patients with autoim-
mune diseases, such as rheumatic disease or inflammatory bowel disease, the risk of autoimmune-related adverse events after using immune checkpoint inhibitors is higher, but adverse events are mostly controlled with glucocorticoids and immunosuppressants and show similar treatment efficacy compared to patients without autoimmune diseases [354,355]. Hence, in cases of mild or well-controlled autoimmune disease, and in cases where flares may not be life-threatening, immune checkpoint inhibitor-based treatment might be considered for patients with pre-existing autoimmune disease [356]. However, there is a lack of data to examine the safety and efficacy of immune checkpoint inhibitors in AIH patients with HCC, as those have been excluded in clinical trials evaluating immune checkpoint inhibitor-based treatment [356]. If autoimmune-related adverse events or AIH flares occur after checkpoint inhibitor-based treatment, it may lead to life-threatening complications, such as liver failure. Therefore, immune checkpoint inhibitor-based therapy should be carefully considered after evaluating the potential risks and benefits.
PROGNOSIS
The 5- and 10-year cumulative incidence rates of death for AIH patients on treatment were 7.1% and 10–32%, while those for liver-related death were 4% and 6–11%, respectively [161,351,357,358]. The 5-, 10-, and 20-year cumulative incidence rates of survival were 90%, 88-91%, and 70%, respectively [8,148]. Standardized mortality ratio (SMR) was 1.63 (95% CI, 1.25–2.02); and when considering liver transplant as “death,” the SMR was 1.86 (95% CI, 1.49–2.26) [148]. According to a single Korean center analysis, the 5- and 10-year overall mortality rates were 6.2% and 12.2% [194], respectively, while the 5- and 10-year cumulative incidence rates of survival were 91.2% and 85.5%, respectively, similar to the prognosis of AIH patients in other countries [200].
Studies from other countries showed that 28–36% of AIH patients had LC and 19% of patients had decompensated LC at the time of diagnosis, while 30–50% of patients without cirrhosis at baseline progressed to cirrhosis eventually, despite immunosuppressive treatment [7,148]. In AIH patients, the presence of cirrhosis is one of the important factors affecting liver disease progression and survival [10,22,24], while age is an important factor associated with cirrhosis [10,60,351]. Elderly patients (age ≥60 years) have a greater frequency of LC at presentation compared to younger patients (age ≤60 patients) (33% vs. 10%, P=0.03) [60]. In addition to cirrhosis and older age, failure to achieve biochemical response within 12 months of treatment, frequent relapse, and non-white ethnicity were also associated with poor prognosis [148,161]. In a Swedish study, AIH patients, including 28.1% of cirrhosis at baseline, had a median time to death of 11.8 years without a significant difference between men and women (8.5 years vs. 13.9 years, P=0.08) [8].
In South Korea, the presence of cirrhosis at the time of AIH diagnosis was found in 12.8–33.5% and decompensated cirrhosis in 4.3% of all cases, which were lower rates than those in other countries [6,34,50,200]. When 53 AIH patients without cirrhosis were followed up for 60 months, 13.2% progressed to cirrhosis, and the 1- and 5-year cumulative incidence rates of cirrhosis and decompensate cirrhosis were 4.7%, 9.8%, 2.9% and 7.6% respectively [50]. A Korean retrospective study including 86 patients with a follow-up duration of 43 months showed disease progression in six patients, including decompensated LC (two patients), HCC (one patient), and liver-related death (three patients) [200].
Risk of hepatocellular carcinoma and extrahepatic malignancy
In Korean AIH patients, HCC was present at the time of diagnosis in 1–3% [6,194]. According to a prospective study conducted in the United Kingdom, during a median follow-up period of 11 years (1–36 years), HCC developed in 6.2% of AIH patients [359-361]. When cirrhosis was present, HCC increased significantly to 12.3% with an annual incidence rate of 1.1–2%. Although the risk of HCC development in AIH patients is lower than in CHB and CHC patients, it is higher compared to the general population [351,358]. According to a meta-analysis, the pooled incidence rate of HCC in AIH patients was 3.54 per 1,000 person-years (95% CI, 2.76–4.55), and the risk was higher in men and cirrhotic patients [362,363]. The risk of developing HCC was higher with older age, longer duration of AIH, and absence of treatment response [364,365]. A higher HCC incidence was observed in Asia compared to Europe and North America [362]. Another meta-analysis with a pooled incidence rate of 3.06 per 1,000 patient-years (95% CI, 2.22–4.23) showed an HCC incidence rate of 10.07 per 1,000 person-years (95% CI, 6.89–14.7) in cirrhotic and 1.14 per 1,000 person-years (95% CI, 0.60–2.17) in non-cirrhotic patients [366]. Further research is required on the need for HCC surveillance in AIH patients without cirrhosis. In contrast, AIH patients with cirrhosis should be considered as a high-risk group; therefore, abdominal sonography and serum AFP measurement should be performed every 6 months [367].
Long-term immunosuppressive therapy in patients with AIH increases the risk of extrahepatic cancer as well as hepatocellular carcinoma [351,358,368]. Long-term immunosuppression results in the derangement of cytokines and subsequent lymphocyte changes leading to defective immune-mediated tumor surveillance control. Apoptosis and proliferation of cancer cells as well as impaired DNA damage and repair mechanism enhance the risk of carcinogenesis [351]. Although the risk of cancer is unknown in Korean AIH patients, the Swedish Cancer Register showed that the 10-year cumulative incidence rates of any cancer (13.6% vs. 9.1%) and extrahepatic cancer (11.2% vs. 8.9%) were higher in AIH patients compared to the general population, and the risk increased in the presence of cirrhosis [369]. AIH patients had a higher 10-year risk of colorectal cancer (1.6% vs. 0.8%), lung cancer (1.9% vs. 0.9%), non-melanoma skin cancer (4% vs. 2.3%), and hematologic cancer (0.7% vs. 0.4%) compared to the matched controls; and for the same cancer types, the use of immunosuppressive treatment was associated with a higher risk [357]. Therefore, AIH patients should adhere to standard guidelines for extrahepatic cancer surveillance.
[Recommendations]
1. HCC surveillance should be performed in AIH patients with LC. (A1)
Notes
Authors’ contribution
List of author contributions is available at the official website of Clinical and Molecular Hepatology (http://www.e-cmh.org).
Conflicts of Interest
Conflicts of interest statement is available at the official website of Clinical and Molecular Hepatology (http://www.ecmh.org).
SUPPLEMENTAL MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
Committee members of KASL Clinical Practice Guidelines for Management of AIH Development Committee
Development timeline of KASL Clinical Practice Guidelines for Management of Autoimmune Hepatitis (2022)
Disclosure of conflict of interests
All committee members who participated in the development of the current guidelines prepared a declaration of interests consisting of the following questions, and information on any conflict of interests within the last 3 years are listed below.
1. Do you have intellectual property rights, such as patents, trademarks, licensing, and royalties, in relation to interventions (drugs, medical technologies, etc.) covered by the clinical practice guidelines?
2. Are you employed (if you have an official/informal title) or have you ever been employed by a company or organization that is commercially related to the interventions covered by the clinical practice guidelines (drugs, medical technology, etc.)?
3. Do you have unlisted shares (stock options, non-trading stocks) or listed shares (over 10 million KRW, including stock options, but excluding indirect investment through mutual funds, etc.) of a company or organization that is commercially related to clinical practice guidelines?
4. Have you ever received research funds, grants, or equipment support from a company or organization that is commercially related to clinical practice guidelines?
5. Have you ever received honoraria, consulting fees, or payments (travel expenses, etc.) exceeding 3 million KRW each year or a total of 10 million KRW or more from companies or organizations that are commercially related to clinical practice guidelines?
6. Do you have any of the relationships described above in your family (parents, spouse, children) or the company in which a family member is involved?
Delphi round agreement on the recommendations
The Delphi Committee rated each recommendation on a 9-point Likert scale from 1 (strongly disagree) to 9 (strongly agree). The mean value of each recommendation was calculated and classified into appropriate (≥7), uncertain (4.0 to <7), or inappropriate (<4). A coefficient of variance ≥0.5 indicated non-consensus and the need for revision. The Delphi Panel agreement on each of the final recommendations is as below.
Abbreviations
6-MP
6-mercaptopurine
6-TG
6-thioguanine
6-TGN
6-thioguanine nucleotides
ACLF
acute on chronic liver failure
AIH
autoimmune hepatitis
AIH-1
autoimmune hepatitis type 1
AIH-2
autoimmune hepatitis type 2
ALF
acute liver failure
ALP
alkaline phosphatase
ALT
alanine aminotransferase
AMA
antimitochondrial antibody
ANA
antinuclear antibody
ANCA
antineutrophil cytoplasmic antibody
anti-LC1
antibody to liver cytosol type 1
anti-LKM1
antibody to liver kidney microsome type 1
anti-SLA
antibody to soluble liver antigen
APRI
aspartate aminotransferase-to-platelet ratio index
AUROC
area under the receiver operating characteristic curve
AZA
azathioprine
CI
confidence interval
CT
computed tomography
DILI
drug-indued liver injury
ELISA
enzyme-linked immunosorbent assay
FIB-4
fibrosis-4 index
FRAX
Fracture Risk Assessment Tool
GGT
gamma-glutamyl transferase
GRADE
Grading of Recommendations
HAI
hepatitis activity index
HLA
human leukocyte antigen
HR
hazard ratio
IFA
indirect immunofluorescence assay
IgG
immunoglobulin G
INR
international normalized ratio
LC
liver cirrhosis
MMF
mycophenolate mofetil
mTOR
mammalian Target of Rapamycin
NUDT15
Nudix hydrolase15
OR
odds ratio
p-ANCA
perinuclear antineutrophil cytoplasmic antibody
p-ANNA
perinuclear antineutrophil nuclear antibody
PBC
primary biliary cholangitis
PSC
primary sclerosing cholangitis
PT
prothrombin time
RR
relative risk
SMA
smooth muscle antibody
SWE
shear wave elastography
TNF
tumor necrosis factor
TPMT
thiopurine S-methyltransferase
UDCA
ursodeoxycholic acid
ULN
upper limit of normal range



