Carbon Ion Radiotherapy in the Treatment of Hepatocellular Carcinoma
Article information
Abstract
Hepatocellular carcinoma (HCC) is a highly lethal cancer with limited treatment options and poor prognosis. Carbon ion radiotherapy (CIRT) has emerged as a promising treatment modality for HCC due to its unique physical and biological properties. CIRT uses carbon ions to target and destroy cancer cells with a high precision and efficacy. The Bragg Peak phenomenon allows precise dose delivery to the tumor while minimizing damage to healthy tissues. In addition, the high relative biological effectiveness of carbon ions can be shown against radioresistant and hypoxic tumor areas. CIRT also offers a shorter treatment schedule than conventional radiotherapy, which increases patient convenience and compliance. The clinical outcomes of CIRT for HCC have shown excellent local control rates with minimal side effects. Considering its physical and biological properties, CIRT may be a viable option for complex clinical scenarios such as patients with poor liver function, large tumors, re-irradiation cases, and tumors close to critical organs. Further research and larger studies are needed to establish definitive indications for CIRT and to compare its efficacy with that of other treatment modalities. Nevertheless, CIRT offers a potential breakthrough in HCC management, providing hope for improved therapeutic outcomes and reduced treatment-related toxicities.
INTRODUCTION
Liver cancer ranks as the eighth and fifth leading cause of cancer-related mortality among females and males, respectively, in the United States [1]. In Korea, it stands as the seventh most prevalent cancer type. With a 5-year survival rate of 38.7%, liver cancer is regarded as one of the fatal types of cancer. Hepatocellular carcinoma (HCC) is the predominant form of primary liver cancer [2]. A multidisciplinary approach has been employed in the management of HCC, with treatment options ranging from surgical resection and liver transplantation to minimally invasive ablation techniques, transarterial therapies, radiation therapy, and systemic therapy [3-5]. The choice of treatment depends on factors, such as disease stage, liver function, and overall patient health, with the ultimate goal of achieving optimal therapeutic outcomes and improving the patient’s quality of life. Due to its high recurrence and poor prognosis rates, the development of effective treatment strategies for HCC has become a pressing. In recent years, carbon ion radiotherapy (CIRT) has emerged as a promising treatment option for patients with HCC, offering potential advantages over traditional modalities such as surgery, chemotherapy, and conventional radiotherapy.
CIRT is a type of particle radiotherapy that utilizes carbon ion beams to precisely target and destroy cancer cells. The key advantages of CIRT are its unique physical and biological properties. Carbon ions deposit more energy within a small volume of tissue, causing more damage to cancer cells and having a better biological effect than photons or protons. Furthermore, carbon ions possess a characteristic dose distribution that enables the delivery of a high dose of radiation to the tumor while minimizing damage to the surrounding healthy tissues. The application of CIRT in the treatment of HCC is based on its ability to overcome some of the challenges posed by conventional therapies.
This article provides an in-depth review on the use of CIRT as a treatment option for HCC. It covers the underlying physical and biological principles of CIRT, clinical outcomes of patients with HCC treated with CIRT, and a comparison of CIRT with other available treatment options. This article also suggested the clinical scenarios where CIRT could yield benefits in the management of HCC.
PHYSICAL ADVANTAGES OF CIRT IN THE TREATMENT OF HCC
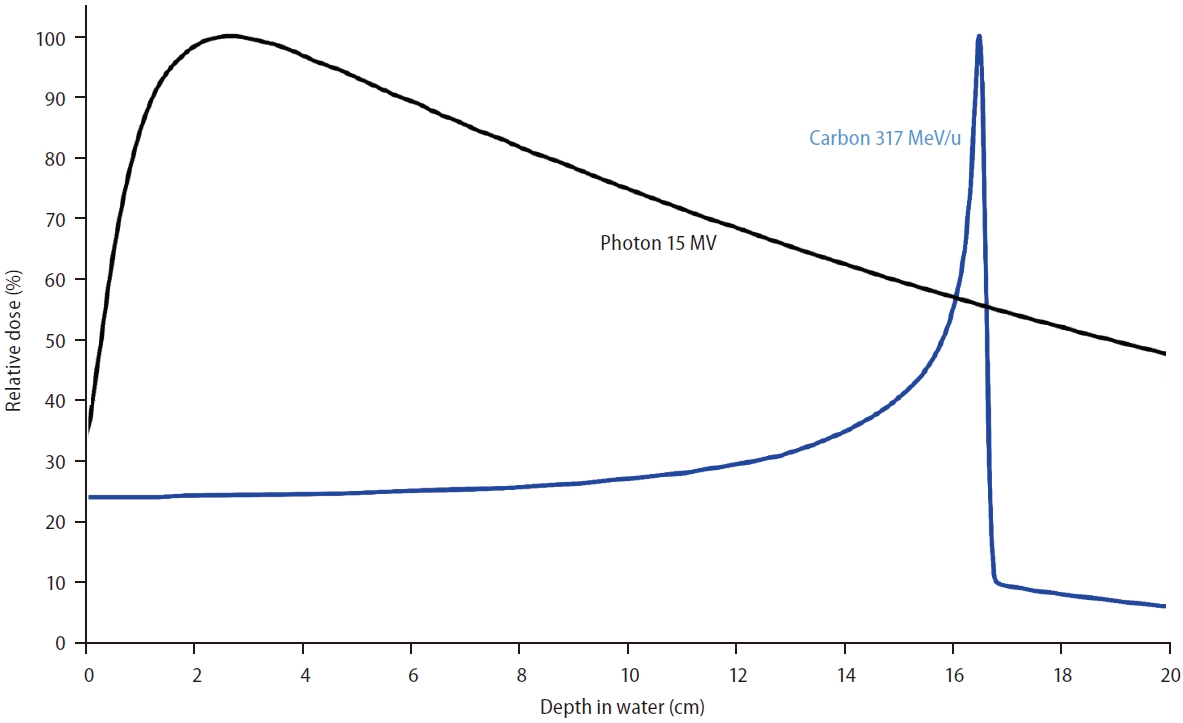
The most notable physical property of the CIRT is the Bragg Peak. This special physical characteristic distinguishes particle therapy from X-ray therapy. As an X-ray beam traverses matter, its intensity gradually decreases owing to the absorption or scattering of photons from the beam. In contrast, when a particle beam passes through matter, it deposits most of its energy in the final millimeters of its trajectory while slowing down. This results in a steep increase in the absorbed dose, known as the Bragg peak (Fig. 1). This characteristic enables precise dose delivery to the target, while minimizing radiation exposure to healthy tissues located before and beyond the target site.
The Spread-Out Bragg Peak (SOBP) concept is employed to treat tumors with larger volumes. Peak energy deposition, the Bragg Peak, occurs at a specific depth within the tissue and can be calculated based on the initial ion energy. The SOBP is generated by superimposing multiple Bragg Peaks of varying energies and intensities, effectively creating a broader and uniform dose distribution within the tumor. SOBP ensures that the entire tumor volume receives a consistent therapeutic dose while preserving the advantageous properties of the Bragg Peak, such as dose conformity and reduced damage to the surrounding healthy tissue. Representative radiation treatment plans for HCC using carbon ion and X-ray beams are shown in Figure 2. Carbon ion beams can produce a more precise and conformal dose distribution to the tumor than X-ray beams while minimizing the exposure of the surrounding normal liver tissue to radiation. In Shiba et al.’s retrospective study [6], a comparison was made between the dosimetric outcomes of patients with HCC who underwent intensity-modulated radiotherapy using X-ray beams and those who received CIRT, both administered at a dose of 60 Gy. The study showed that patients treated with CIRT had significantly lower mean liver doses and a lower percentage of normal liver volume exposed to radiation doses exceeding 5 Gy, 10 Gy, 20 Gy, 30 Gy, 40 Gy, and 50 Gy than those who underwent intensity-modulated radiotherapy. Patients with HCC often present with compromised liver function, which makes the protection of healthy liver tissue a crucial aspect of HCC treatment. Therefore, this feature makes CIRT a favorable option for HCC management.

Representative radiation treatment plans for hepatocellular carcinoma using carbon ion beams (A) and X-rays (B). Note that carbon ion beams can produce a more precise and conformal dose distribution to the tumor than X-ray beams, while minimizing the exposure of the surrounding normal liver tissue to radiation.
BIOLOGICAL ADVANTAGES OF CIRT IN THE TREATMENT OF HCC
Strong biological effectiveness
Linear energy transfer (LET) refers to the rate of energy loss experienced by particle beams as they penetrate tissues. Photons, electrons, and protons beams are low-LET radiations that exhibit sparse ionization. Carbon ions, alpha particles and fast neutrons beams are high-LET radiations that exhibit dense ionization. Heavy ions, such as carbon ions, have a high atomic mass and possess a high LET. The LET and relative biological effectiveness (RBE) of radiation are closely associated. High-LET radiation tends to have a higher RBE than low-LET radiations. The RBE is defined as the ratio of the doses required by two different types of radiation to cause the same level of effect. The RBE of photons is set at 1. In proton therapy, a consistent RBE value of 1.1 is widely accepted. In contrast, the RBE of carbon ions is not a constant value, but rather depends on their position within the treatment beam. As the carbon ions penetrate further into the target lesion, their RBE increases. These features offer therapeutic benefits because the biological effects of carbon ion beams intensify as they progress deeper into the tumor area. The local RBE values for carbon ions can reach as high as 2.0–3.5. Moreover, at the entry site (normal tissue), the RBE value is lower than that of the target lesions. This disparity in RBE between cancerous and normal tissue expands the therapeutic window, as it enhances the biological effects within the target region while minimizing damage to normal tissue.
Strong effect on hypoxic tumor
Tumor hypoxia has been recognized as a key mechanism that causes radioresistance in cancer cells. Chronic hypoxia results from excessive proliferation of cancer cells accompanied by poor vasculature. The increased distance between cells and their nearest blood vessels restricts oxygen diffusion from the tumor microvessels to the surrounding tissue. Low-LET radiation predominantly induces DNA damage by generating free radicals; this is known as the indirect action of radiation. The generation of free radicals is promoted by the presence of oxygen, whereas high-LET radiation directly strikes the DNA molecule, disrupting its molecular structure; this is called the direct action of radiation. This extensive damage caused by high-LET radiation is less dependent on the oxygen concentration. Consequently, carbon ion beams demonstrate superior efficacy in hypoxic tumors. Given the common occurrence of hypoxia in the intratumor regions of HCC, caused by abnormal microvasculature and uncontrolled cell proliferation leading to low oxygen levels, CIRT can offer valuable benefits for managing HCC. Hypoxia in HCC is known to be associated with tumor aggressiveness, chemoresistance, and immunotherapy resistance, making CIRT’s superior effectiveness in hypoxic conditions relevant for HCC treatment [7].
Short treatment schedule
Fractionated irradiation is an important concept in conventional radiotherapy that uses X-rays. Several biological effects contribute to the advantages of fractionated radiation. Between irradiations, damaged normal tissues recover as sublethal cell damage is repaired (repair) and the cells repopulate (repopulation). Additionally, the time between irradiation sessions allows tumor cells to progress into the radiosensitive phases of the cell cycle (redistribution) and allows the surviving hypoxic tumor cells to become oxygenated (reoxygenation). The “4Rs”—repair, repopulation, redistribution, and reoxygenation—form the fundamental rationale for radiation fractionation.
However, the 4Rs have diminished significance for high-LET beams, including CIRT. For example, sublethal damage repair and repopulation of the tissue is suppressed in CIRT. Carbon ion beams are less affected by the cell cycle or cellular oxygenation than X-rays. The implications of the 4Rs, and thereby the effect of fractionated irradiation, on CIRT are minor compared to those of conventional X-ray therapy. Furthermore, due to the sharper physical dose distribution of CIRT, critical organs are exposed to reduced radiation doses, allowing for CIRT hypo‐fractionation strategies. The application of hypofractionated radiotherapy in HCC has benefits in several aspects. In X-ray therapy, the reduction of fractionation, known as stereotactic body radiotherapy, has been shown to not only have clinical anti-tumor effects but also minimize the impact of radiation-induced lymphopenia in HCC [8]. Furthermore, hypofractionated radiotherapy leads to a significant activation of the immune system, thereby enhancing the efficacy of immune checkpoint inhibitors in HCC treatment [9,10]. To implement hypofractionated radiotherapy, ensuring safety is crucial, and CIRT’s physical and biological characteristics offer the necessary assurance in this regard.
Historically, CIRT has implemented fewer fractions than conventional X-ray therapy. In general, conventional radiotherapy using X-ray or proton beam therapy uses a larger number of scheduled fractions, ranging from 10 to 35 in published reports [11,12]. In contrast, current CIRT protocols mostly use 2 or 4 fractions for tumors located at a distance from the gastrointestinal tracts, while 12 fractions or more were typically used for tumors in close proximity to the gastrointestinal tracts (Table 1). In a multi-institutional retrospective study conducted by the Japan Carbon Ion Radiation Oncology Study Group, short course CIRT with 2 or 4 fraction regimens has demonstrated a curative local effect while maintaining acceptable treatment-related toxicities for HCC [13].
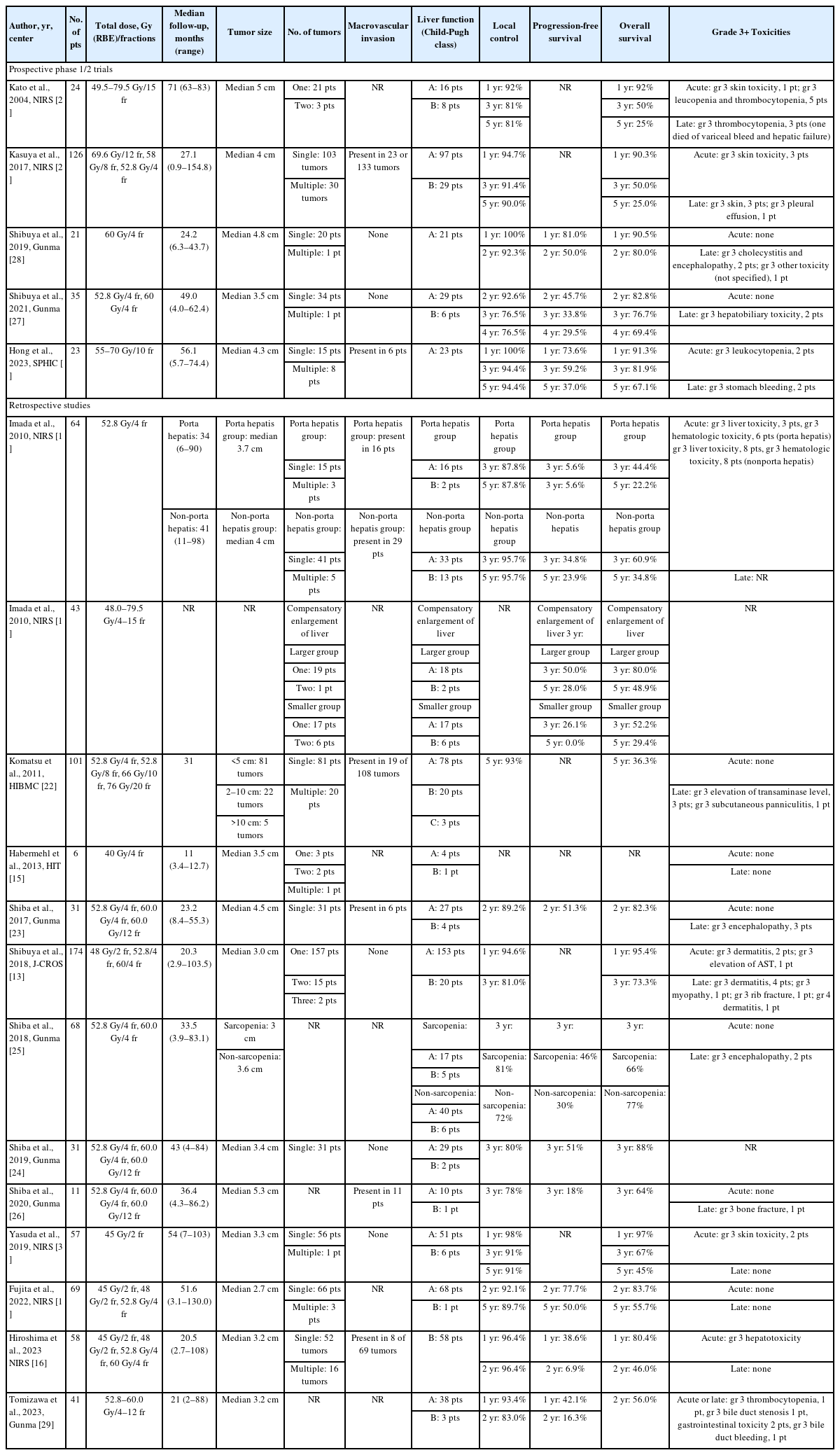
Literature review of studies reporting outcomes after carbon ion radiotherapy for hepatocellular carcinoma
In summary, the physical properties of CIRT, specifically, the superior dose distribution resulting from the Bragg Peak, enable a high dose concentration in the tumor area while minimizing radiation exposure to normal organs. Furthermore, biological properties such as high RBE within the target area and low RBE in non-target areas expand the therapeutic window of carbon-ion beams in comparison to proton and photon beams. The efficacy of CIRT in addressing tumor hypoxia helps overcome radioresistance and aid in controlling large hypoxic tumors. Additionally, a shorter treatment schedule due to the fewer fractions enhances patient convenience and increases compliance in patients with comorbidities or those who must travel long distances for treatment.
CLINICAL OUTCOMES OF CIRT FOR HCC
Treatment outcomes of CIRT for HCC
The efficacy and feasibility of CIRT for HCC have been investigated in several prospective phase I and II studies (Table 1) [13-30]. The first prospective phase I trial was reported from the National Institute of Radiological Sciences in 2004 exploring dose escalation from 49.5 Gy (RBE) in 15 fractions to 79.5 Gy (RBE) in 15 fractions [21]. No severe adverse effects or treatment-related deaths were reported. The local control rate was 81% at both 3 and 5 years. The National Institute of Radiological Sciences reported the combined results of phase I and II trials in 2017 [20]. The maximum tolerated doses were determined at 69.6, 58.0, and 52.8 Gy (RBE) in 12, 8, and 4 fractions, respectively, and 52.8 Gy (RBE) in 4 fractions was established as the recommended dose regimen for the two phase II studies. Gunma University has reported the results of prospective trials conducted in 2019 and 2022 [27,28]. Regimens of 52.8 Gy (RBE) and 60 Gy (RBE) in four fractions were used and showed 92.3% local control rate at 2 years and 76.5% local control rate at 4 years. Late grade 3 hepatobiliary toxicity occurred in 2 patients with no grade 4 or more toxicity. The Shanghai Proton and Heavy Ion Center has reported the outcomes of a phase I trial. Dose levels ranged from 55 to 70 Gy (RBE) in 10 fractions, and no dose-limiting toxicity was observed. The 5-year local control rate was 94.4% [17].
Several retrospective studies regarding CIRT for HCC have been also conducted (Table 1) [13-30]. A multi-institutional retrospective study conducted by the Japan Carbon Ion Radiation Oncology Study Group showed the results of 174 patients with HCC treated with CIRT with regimens of 48 Gy (RBE) in two fractions, 52.8 Gy (RBE) in 4 fractions, or 60 Gy (RBE) in 4 fractions [13]. The 3-year local control rate was 81.0% and the 3-year overall survival rate was 73.3%. Acute grade 3 toxicities included dermatitis in two patients and elevation of AST in one patient. Late grade 3 toxicities included dermatitis in four patients, myopathy in one patient, and rib fractures in one patient. Grade 4 late dermatitis occurred in one patient. Upon reviewing the retrospective studies outlined in Table 1, the local control rate at 5 years was generally close to or more than 90% overall. Acute or late toxicities of grade 3 or higher were observed in a few cases. The recent series uses 2 or 4 fractions for tumors distant from gastrointestinal tracts and 12 or more fractions for tumors close to them, optimizing dose delivery and minimizing adverse effects.
CIRT in comparison with other modalities
Most existing literature focuses solely on CIRT. A few small retrospective studies have examined the effectiveness of CIRT compared with other treatment modalities. Further studies with larger numbers of patients or prospective designs are needed to directly compare CIRT with other modalities.
Shiba et al. [24] compared CIRT with transarterial chemoembolization for the treatment of single HCC using propensity score matching. After analyzing 17 matched pairs, the 3-year overall survival, local control, and progression-free survival rates in the CIRT vs. transarterial chemoembolization groups were 88% vs. 58% (P<0.05), 80% vs. 26% (P<0.01), and 51% vs. 15% (P<0.05), respectively. These results revealed that CIRT resulted in more favorable clinical outcomes than transarterial chemoembolization, although larger patient numbers are required to confirm the results.
Fujita et al. [14] compared CIRT with radiofrequency ablation as initial treatments for early-stage HCC. Among the 560 patients examined, 69 underwent CIRT and 491 underwent radiofrequency ablation. After propensity score matching, the CIRT group had significantly lower cumulative intrasubsegmental recurrence rates than the radiofrequency ablation (2- year, 12.6% vs. 31.7%; 5-year, 15.5% vs. 49.6%, P=0.004). However, local recurrence rates, progression-free survival, and overall survival were comparable between the two groups. Notably, no adverse events grade 3 or higher were observed in the CIRT group, while 1.2% of patients showed grade 3 adverse events in the radiofrequency ablation group.
Suggested special scenarios for the application of CIRT in HCC
Although definitive indications for CIRT in HCC have yet to be established, its superior dose profiles make CIRT a viable choice in complex clinical cases that are unsuitable for traditional X-ray therapy. In particular, CIRT can reduce radiationrelated hepatotoxicity while maintaining effective tumor control. Radiation-induced liver disease (RILD) is a form of subacute liver injury triggered by radiation and is one of the most dreaded complications of radiotherapy for liver cancer [31]. To minimize the risk of RILD, stereotactic body radiation therapy and conventional radiotherapy employ liver dose constraints [32,33]. RILD is one of major hurdles for radiation dose escalation in HCC treatment because adherence to liver dose constraints is crucial during the radiotherapy planning process. Previous studies have identified pretreatment liver function, radiation dose, and irradiated liver volume as risk factors for RILD [34-36]. Thus, in situations where substantial hepatotoxicity is anticipated with photon radiotherapy, CIRT presents an advantageous alternative. The suggested scenarios for the application of CIRT in HCC are patients with poor liver function, large tumors, patients who require re-irradiation, and patients with HCC close to critical organs.
CIRT offers a promising alternative treatment for patients with poor liver function, who are often ineligible for radiotherapy or other local therapies. Hiroshima et al. [16] demonstrated this in their study of 58 patients with HCC with Child-Pugh B liver function. Only one patient experienced grade 3 acute hepatotoxicity with no acute or late grade 4 or higher adverse events following CIRT administered at doses of 45 Gy (RBE) or 48 Gy (RBE)/2 fractions, as well as 52.8 Gy (RBE) or 60 Gy (RBE) in 4 fractions.
CIRT also offers advantages for the treatment of patients with large tumors. In general, as the tumor size increases, the radiation target volume also increases, as does the radiation exposure of the normal liver. In addition, large tumors often show a poorer response to radiation than small tumors. A retrospective study revealed that the response rates to stereotactic body radiation therapy using X-rays for HCC <4 cm, 4–10 cm, and >10 cm were 96.15%, 90.90%, and 76.47%, respectively [37]. Given its superior dose distribution and high RBE, CIRT may significantly improve the treatment outcomes in large HCCs, while minimize the risk of RILD.
In cases requiring re-irradiation, the cumulative radiation dose to the liver increases when considering both the previous radiotherapy and the re-irradiation doses. As the cumulative mean radiation dose to the liver increases, the risk of hepatotoxicity also increases [38]. CIRT can reduce the radiation dose to the normal liver during re-irradiation by providing excellent radiation dose distribution. Tomizawa et al. [29] reported no instances of grade 4 or higher toxicity among 41 patients who underwent repeat CIRT for intrahepatic HCC recurrence. The prescribed dose was 52.8 to 60.0 Gy (RBE) in 4 to 12 fractions. The change in the albumin-bilirubin score before and after the second CIRT was also insignificant, suggesting minimal liver function deterioration after re-irradiation using CIRT.
Another beneficial scenario for CIRT is when HCC is in close proximity to a critical organ. For instance, HCC in the caudate lobe typically has a poor prognosis owing to its proximity to the portal trunk and inferior vena cava, which facilitates early systemic spread. Additionally, its deep location in the liver and proximity to major vessels pose technical challenges for surgical resection, radiofrequency ablation, and ethanol injection in the caudate lobe [39]. Furthermore, the complex arterial blood supply of the caudate lobe makes transarterial chemoembolization difficult to achieve local tumor control [40]. In such cases, radiotherapy can be an effective local treatment option for HCC in the caudate lobe, being less influenced by the anatomical features of the caudate lobe. Various radiotherapy techniques, such as intensity-modulated radiotherapy, stereotactic body radiotherapy, and particle beam therapy, have been attempted for patients with difficult-to-treat HCC with other local therapy modalities [41]. CIRT has also been applied in situations where HCC is located in the caudate lobe. Okazaki et al. [42] reported the results of CIRT in the treatment of HCC located in the caudate lobe. The study demonstrated no local recurrence, and only two instances of grade 2 or 3 late adverse events were observed among the nine patients. Furthermore, for HCCs adjacent to the porta hepatis, anatomical resection can be invasive because of its large resection volume. CIRT has demonstrated excellent outcomes when applied in situations where the HCC is close to the porta hepatis. Imada et al. [19] compared the CIRT results between patients with HCC located within 2 cm of the main portal vein and those with HCC far from the porta hepatis. Their findings revealed no significant differences in overall survival, local control, or toxicity between the two groups, highlighting the effectiveness and safety of CIRT in the porta hepatis group just as in the non-porta hepatis group. Notably, biliary stricture associated with CIRT was not observed.
LIMITATIONS OF CIRT IN HCC
One primary limitation is the scarcity of clinical evidence comparing the CIRT to other treatment modalities [43]. As CIRT is a relatively new and specialized approach, there are limited large-scale clinical trials for HCC. This lack of data hinders a comprehensive assessment of its long-term effectiveness and safety in treating diseases.
Another challenge arises when HCC is located near the gastrointestinal tract. The highly conformal radiation field produced by carbon-ion beams is affected by various uncertainties, including bowel motion and bowel gas. There is a possibility that focal high dose can affect the gastrointestinal mucosa, potentially leading to complications such as ulceration, bleeding, and perforation [44]. Although studies have shown low occurrences of gastrointestinal complications with CIRT, there remains a potential risk due to the impact of high-intensity doses.
Moreover, CIRT’s physical and biological properties present further limitations. Carbon ion beams have a more rapid lateral fall-off around the target volume compared to proton beams, resulting in smaller lateral penumbra. However, beyond the distal end of the peak, carbon ion beams exhibit a fragmentation tail caused by a small dose deposited due to nuclear interactions and particle fragmentation, whereas proton beams show almost no dose deposition [45]. Since the tail contains only fragments with a low atomic number, the biological effect of this fragmentation tail and its clinical implications are minimal.
Range uncertainty in the beam path length is another major concern. The stopping position of the carbon beam is sensitive to density variations along the beam path. Due to the steep dose gradient, anatomical changes, including organ movements or the changes in bowel gas, can significantly impact the robustness of the treatment plan. To mitigate these uncertainties, robust treatment planning and motion management techniques have been developed [46].
Furthermore, RBE of CIRT possesses uncertainty. RBE is affected by numerous factors, including measured endpoint, dose, dose rate, dose per fractionation, number of fractions, particle charge and velocity, oxygen concentration, and cell-cycle phase. While biophysical models such as the local effect model (LEM) or microdosimetric kinetic model (MKM) are used to determine the RBE of CIRT, theoretical modeling of the biological effects of heavy ions remains a challenging task due to the complexity and limited knowledge of the physical, chemical, and biological processes involved [47]. Despite CIRT’s successful application for several decades in the real world, research on these biophysical models continues to be an active area of investigation [48].
CONCLUSION
The traditional treatment approaches for HCC often exhibit limited efficacy and substantial side effects. CIRT is an advantageous solution owing to its unique physical and biological properties. The Bragg Peak, a key attribute of CIRT, enables precise delivery of high-dose radiation to the tumor site while minimizing exposure to the surrounding healthy tissue. Furthermore, CIRT’s high LET contributes to an elevated RBE. This capability enhances the destruction of cancer cells, particularly in hypoxic tumors that tend to resist traditional radiotherapy. Several prospective and retrospective studies have demonstrated the benefits of CIRT for HCC management. Compared with conventional therapies, CIRT exhibits excellent local control and reduces adverse effects. Its effectiveness in treating larger tumors along with its suitability for patients with compromised liver function, those requiring reirradiation, or those with tumors located near the clinical organs, further highlights CIRT’s potential as a groundbreaking therapeutic strategy. However, despite the promising results from prospective I/II and small retrospective studies, it is essential to acknowledge the current lack of phase III clinical trials directly comparing CIRT with other treatment modalities in HCC. The superiority of CIRT in effectiveness and safety over conventional therapies has not yet been definitively demonstrated. Well-designed phase III clinical trials are warranted in the future to provide robust evidence and establish CIRT as a leading therapeutic option for HCC. Through these endeavors, we can establish more definitive guidelines for the implementation of CIRT in HCC treatment, paving the way for improved patient outcomes.
Notes
Authors’ contribution
Conception, design of the study, and literature review and analysis: HK Byun and J Seong; drafting and critical revision and editing, and final approval of the final version: HK Byun, C Kim and J Seong.
Conflicts of Interest
The authors have no conflicts to disclose.
Abbreviations
CIRT
carbon ion radiotherapy
HCC
hepatocellular carcinoma
LET
linear energy transfer
RBE
relative biological effectiveness
RILD
radiation-induced liver disease
SOBP
spread-out Bragg peak