Current evidence and the potential role of proton beam therapy for hepatocellular carcinoma
Article information
Abstract
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related death, and external beam radiation therapy has emerged as a promising approach for managing HCC. Proton beam therapy (PBT) offers dosimetric advantages over X-ray therapy, with superior physical properties known as the Bragg peak. PBT holds promise for reducing hepatotoxicity and allowing safe dose-escalation to the tumor. It has been tried in various clinical conditions and has shown promising local tumor control and survival outcomes. A recent phase III trial demonstrated the non-inferiority of PBT in local tumor control compared to current standard radiofrequency ablation in early-stage HCC. PBT also tended to show more favorable outcomes compared to transarterial chemoembolization in the intermediate stage, and has proven effective in-field disease control and safe toxicity profiles in advanced HCC. In this review, we discuss the rationale, clinical studies, optimal indication, and future directions of PBT in HCC treatment.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common primary malignancy arising in the liver and is increasing in incidence with significant impacts on morbidity and mortality [1]. While surgical management remains the primary treatment option, the majority of patients are not appropriate candidates due to advanced disease at diagnosis or poor expected postoperative liver function or surgical morbidity. This renders alternative local treatments critical for the long-term management of these patients in a variety of settings. Nonsurgical treatment options include percutaneous ablation, transarterial chemoembolization (TACE), selective internal radiotherapy (SIRT), and external beam radiation therapy (EBRT). EBRT modalities include 3D conformal radiotherapy (3D-CRT), intensity-modulated radiation therapy (IMRT), volumetric-modulated arc therapy (VMAT), stereotactic body radiation therapy (SBRT), proton beam therapy (PBT), and carbon ion radiotherapy (CIRT). Because key risk factors for the development of HCC include cirrhosis of any cause and hepatitis B or C virus infections, EBRT has had a very limited role in the treatment of HCC in patients whose livers are mostly cirrhotic or poorly functioning, as these patients are most vulnerable to radiation-induced liver disease (RILD) [2,3]. With recent advances in EBRT technology, photon EBRT such as IMRT demonstrated clinical efficacy without increasing RILD in the management of HCC, and the role of PBT has emerged as a powerful technique with its superior physical property in ablative dose delivery to tumors while sparing the uninvolved liver and other nearby critical organs. Here, we review the current evidences and potential roles of PBT according to the Barcelona Clinic Liver Cancer (BCLC) staging classifications [4] in the management of HCC
RATIONALE AND DOSIMETRIC BENEFIT OF PBT
The liver is one of the important radiation dose-limiting organs during EBRT, with RILD risk associated with the irradiated liver volume [5-7]. Thus, minimizing the radiation dose to the remaining normal liver during EBRT for HCC is crucial. Conceptually, more sophisticated EBRT techniques, including IMRT, VMAT, and PBT, may improve tumor control in HCC patients by delivering a high radiation dose to the tumor while minimizing the dose to the remaining normal liver, thereby minimizing impairment of the remaining hepatic reserve.
Regarding the physical characteristic, the X-ray dose delivered decreases gradually along the beam path with increasing beam depth [8]. Thus, an exit dose is inevitably delivered to adjacent normal tissues, and even IMRT or VMAT cannot avoid low-dose delivery at the distal area of the beam path. In contrast, a proton beam has a finite range of energy deposition and loses most of its energy within a very short distance at the end of the beam range. This results in a sharp rise and fall in energy absorption, known as the Bragg peak. Therefore, PBT has been considered to have superior physical properties compared to other X-ray-based EBRT techniques, delivering a high radiation dose to the tumor while minimizing the radiation dose delivered to the remaining normal liver, thereby minimizing impairment of the remaining hepatic reserve. Figure 1 presents the radiation dose distribution of various treatment plans using X-ray and PBT for representative 5.7 cm sized HCC case in segment 8. PBT showed the advantage of less radiation exposure in the remaining normal liver, especially in the low-dose area. Regarding the effect of PBT according to tumor size, previous dosimetric analyses have demonstrated that the larger the tumor size, the greater the benefit of PBT in decreasing the risk of RILD [9,10].
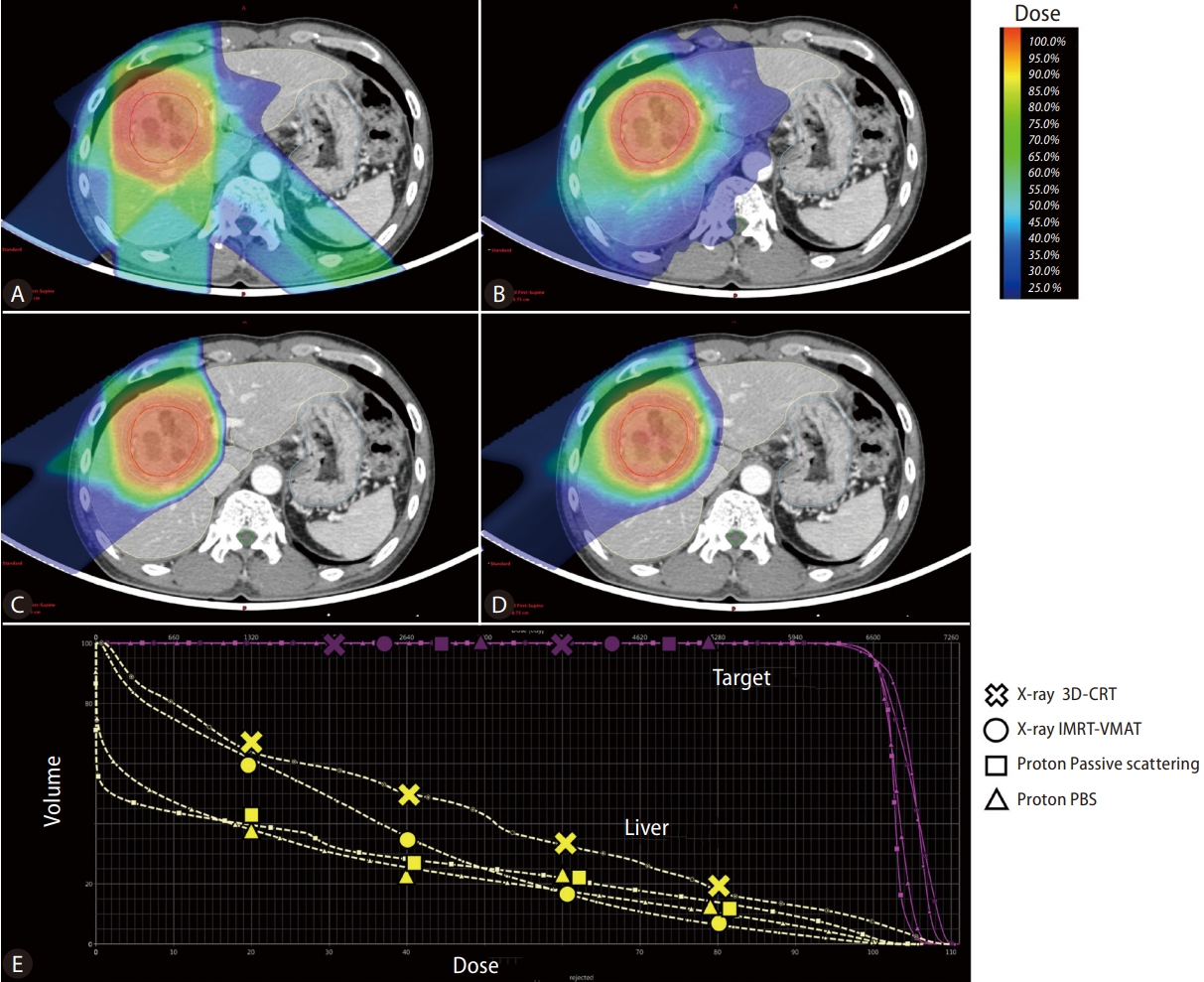
Radiation dose distributions of treatment plans for hepatocellular carcinoma using X-ray 3D-conformal RT (A). X-ray intensity modulated RT-volumetric modulated arc therapy (B). Proton beam therapy-passive scattering (C). Proton beam therapy-pencil beam canning (D) and the Dose-volume histogram graph of each technique (E). PBS, pencil beam scanning; IMRT, intensity modulated radiation therapy; VMAT, volumetric-modulated arc therapy.
Several dosimetric comparison studies have demonstrated the dose volumetric benefits of PBT compared to 3D-CRT and/or IMRT for HCC. Li et al. [11] found that PBT reduced the mean liver dose (Dmean), the fractional volume of the liver receiving doses greater or equal to 10 Gy (V10), 20 Gy (V20), 30 Gy (V30) and better spared non-liver organ-at-risks (OAR) (stomach and kidney) than 3D-CRT or IMRT. Wang et al. [12] demonstrated similar results, with significant reductions in the V30 of the remaining normal liver and the Dmean, as well as reduced stomach, duodenum, heart, and spinal cord by PBT compared to IMRT. Kim et al. [13] compared dose-volume histogram data among helical-IMRT (H-IMRT), VMAT, and PBT for HCC and found that PBT provided equal planning target volume (PTV) tumor coverage, conformity index, and homogeneity index values and significantly better sparing of the liver (Dmean and V5 to V35 for remaining normal liver) and non-liver OARs (D2 cm3 of the stomach and spinal cord) compared to H-IMRT and VMAT. Even though the difference between PBT and either H-IMRT or VMAT in the irradiated volumes of the remaining normal liver at higher doses (from V45 to V55) may not have been clinically significant (less than 3%), PBT significantly reduced the irradiated liver volume at dose levels below V35 (about 50% of the prescribed dose) [13]. These data suggest that PBT may be superior to other EBRT modalities, including 3D-CRT, H-IMRT, and VMAT, in reducing the risk of RILD, and it may also have an advantage during dose escalation.
PROTON BEAM THERAPY TECHNIQUES
Numerous published data demonstrating the efficacy of PBT for HCC have utilized passive scattering (PS)-PBT treatment techniques. Pencil beam scanning (PBS)-PBT may provide more conformal dose distribution and superior normal tissue sparing by the intensity modulation of PBT, enabling improved dose optimization and conformity along the proximal edge compared to PS-PBT [14,15]. PBS-PBT is considered more sensitive to organ motion with respiration than passive scattering due to the interplay effect [15]. Recently, published retrospective series demonstrated the feasibility, safety, and efficacy of PBS-PBT for treating HCC. Although only small retrospective series have been reported, most studies achieved high local tumor control (around 95% at one year) and low toxicity profiles [16,17]. Yoo et al. [18] compared the outcomes of HCC patients treated with either PS-PBT or PBS-PBT. After propensity score matching, they revealed no difference in toxicity, tumor control, or survival between patients treated with PS-PBT or PBS-PBT [18]. As a majority of newer PBT centers have PBS-PBT capability, an increase in data supporting PBS-PT for HCC treatment is anticipated.
RE-IRRADIATION
In selected cases, PBT can be the optimal EBRT technique for delivering a second course of radiation with a curative dose to a previously irradiated liver by preserving the remaining healthy liver and the adjacent critical abdominal organs [19]. Early reports of Hashimoto et al. [20] published in 2006 demonstrated the safety and feasibility of proton re-irradiation for recurrent HCC patients (n=27), and treatment-related toxicities of grade 3 or higher were observed in about 18% (n=5), of which 2 developed acute hepatic failure and the remaining 3 had late injuries (1 rib fracture and 1 bile duct stenosis). McDuff et al. [21] (n=49) reported the more recent experiences confirming the safety and efficacy of proton re-irradiation for liver malignancies with the median follow-up of 10.5 months since re-irradiation. With regard to toxicity, only 2 patients (4.1%) experienced grade 3 toxicity of radiation-induced liver disease after reirradiation. Oshiro et al. [22] retrospectively assessed 83 patients who received liver re-irradiation with protons, including 15 patients treated with three or more definitive EBRT courses. For most patients, repetitive PBT is the only possible treatment option because of the lack of other local therapeutic options. Patients were treated with similar PBT doses of 70 gray-equivalent (GyE) at re-irradiation, following an initial course of 71 GyE. The most commonly used dose schemes were 72.6 GyE in 22 fractions (33%), 66 GyE in 10 fractions (33%), and 74 Gy in 27 fractions (19%) (mean dose per fraction 3.3 GyE). Second-course planning considerations generally included maintaining a normal liver volume of more than 500 mL. This second course of PBT with definitive doses resulted in a median OS of 61 months from the time of the first treatment course, with no severe radiation-induced liver dysfunction or other acute toxicities [22].
COMPARISON WITH OTHER EBRT TECHNIQUES
While PBT has been established as an acceptable local therapeutic option for early-to-advanced HCC, there still is a lack of high-quality evidence to guide recommendations for when proton therapy should be clearly preferred over photon therapy. Cheng et al. [23] compared 110 HCC patients treated with either photon EBRT (n=55) or PBT (n=55) with curative intent after propensity matching. About half of the patients had vascular invasion. Cox regression analysis revealed a significant survival benefit (P=0.032, hazard ratio [HR]=0.56, 95% confidence interval [CI]: 0.33–0.96) and lower risk of RILD (11.8% vs. 36%, P=0.004) in the PBT group compared to the photon group [23]. Qi et al. [24] performed a meta-analysis to compare charged particle therapy versus photon therapy for HCC patients. The pooled OS was significantly higher for charged particle therapy than for conventional photon EBRT with improvements in progression-free survival and local control, while comparable efficacy was found between charged particle therapy and SBRT in OS, progression-free survival and local control. High-grade acute and late toxicity associated with charged particle therapy was lower than that of conventional photon EBRT and SBRT [24]. Hasan et al. [25] compared PBT (n=71) with SBRT (n=918) in stage I-II HCC patients in the National Cancer Database. The results showed that PBT was independently associated with longer survival than SBRT (HR=0.48, 95% CI: 0.29–0.78), despite being delivered to HCC patients with multiple poor prognostic factors [25]. NRG GI003 (NCT03186898) is an ongoing randomized trial to compare PBT versus conventional photon EBRT for HCC.
CIRT, a type of particle beam therapy, is known to have potential advantages with higher radiobiological effectiveness (RBE), offering therapeutic benefits in hypoxic or radioresistant tumor cells [26]. Thus, CIRT has broadened its clinical application and was recently attempted in HCC. Despite the potential benefits of CIRT with high RBE values, the clinical outcome of CIRT appears to be similar to that of PBT, with about 80–90% local tumor control and 50% OS at three years [27,28].
From a treatment planning perspective, dosimetric studies suggest that PBT is advantageous for normal liver-sparing in patients with tumors >3 cm in specific locations or for larger tumors (>5 cm) in patients with good baseline liver function [9,29,30]. For patients with deteriorated liver function, the indication for PBT should be more generous. Additional insight can be gained from the consensus report from the Miami Liver Proton Therapy Conference held in 2019 [31]. This expert panel recommended that proton therapy be strongly considered in the following scenarios: i) normal liver dose constraints cannot be met with photon therapy; ii) Child-Pugh B or greater cirrhosis based on data showing that low doses to the normal liver are associated with hepatotoxicity [32] and favorable outcomes with PBT [33]; iii) larger tumor size; iv) smaller uninvolved liver volume (e.g., <800 cm3), common in more severely cirrhotic patients and after partial liver resection; v) high tumor-to-liver ratio; vi) multiple number of tumors; and vii) prior radiation therapy to the liver.
CLINICAL BENEFIT OF PBT FOR HEPATOCELLULAR CARCINOMA
Early-to-intermediate-stage HCC (BCLC stage 0-B)
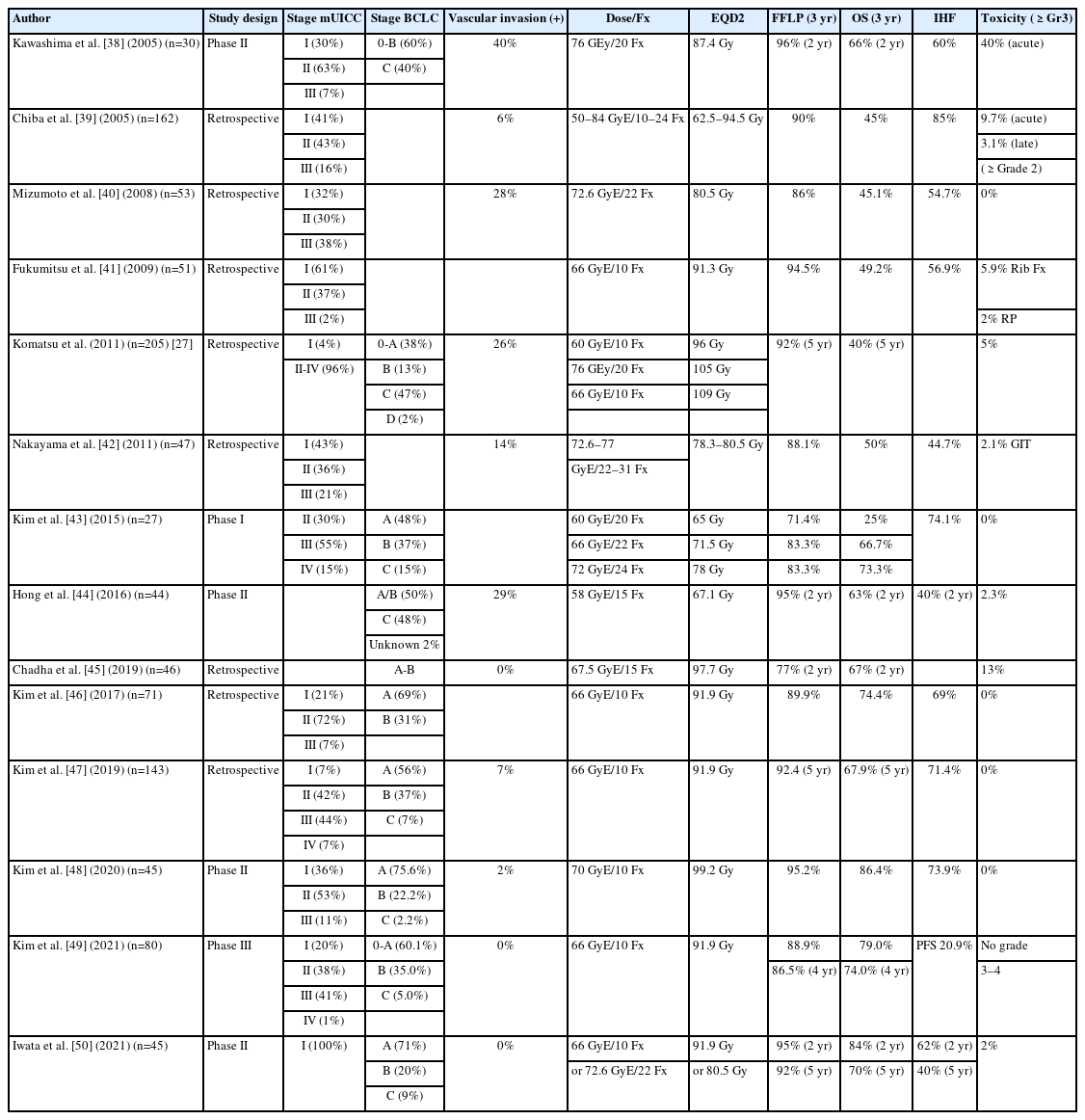
Early-to-intermediate-stage HCC encompasses the largest subgroup of patients with HCC. The recommended early-stage treatments are surgical resection, ablation, and transplantation, and the expected five-year overall survival (OS) rate ranges from 50 to 70% [4,34-36]. The standard therapeutic approach for patients with intermediate-stage disease is TACE, and the expected median survival time is about 30 months [4,37]. PBT demonstrated an excellent local control rate reaching 85–95% and a comparable OS rate of more than 50% at 3–5 years after PBT in patients who received the current standard treatment for early-to-intermediate-stage HCC (Table 1) [27,38-50].
PBT may have several advantages, such as non-invasiveness, use in locations unsuitable for other therapy, and non-echogenicity tumor compared to other local treatments, such as surgical resection and percutaneous ablative therapy, including radiofrequency ablation (RFA). The first phase 3 randomized controlled trial compared PBT and RFA in small recurrent/residual HCC (size <3 cm, number ≤2) and demonstrated that the local control effect of PBT was not inferior to that of RFA (PBT 86.5% vs. RFA 78.3% at 4-year, P=0.114) in HCC patients [49]. Crossover was allowed if the assigned treatment was technically infeasible, and PBT showed better feasibility than RFA with significantly lower crossover rate (8.3% vs. 26.4%, P=0.004). The most common treatment-related toxicity was radiation pneumonitis (32.5%) for PBT and increased alanine aminotransferase levels (96.4%) and abdominal pain (30.4%) for RFA, respectively. PBT was tolerable and safe, consistent with the known profile. The associated good feasibility and comparable clinical outcomes suggest that PBT may be a promising treatment option for small HCCs. TACE is a common treatment option for intermediate-stage or multiple HCCs, but local tumor control is often disturbed by the complex arterial blood supply of the tumor, such as collaterals [51]. In general, tumor control by PBT is not compromised by the complexity of the tumor blood supply. Bush et al. [52] recently reported their final results of a prospective randomized clinical trial comparing PBT with TACE in unresectable HCC. PBT was associated with better PFS and LC compared to TACE and even associated with fewer posttreatment hospitalization days, and reduced cost of treatment.
PBT seems to have excellent long-term local tumor control in treatment-naïve HCC patients, ranging from 87% to 94%, with an OS rate ranging from 66 to 69%, which is comparable to other recommended first-line treatments. Fukuda et al. [53] reported 5-year outcomes in 129 treatment-naïve HCC patients after delivering a total of 66.0–77.0 GyE of PBT in 10–35 fractions. The 5-year local tumor control and OS rates were 94% and 69% for patients with 0/A stage disease (n=9/21) and 87% and 66% for patients with B-stage disease (n=34), respectively. Kim et al. [54] reported the clinical outcomes of 46 treatment-naïve HCC patients treated with PBT and showed similar results, with a 5-year freedom from local progression rate of 92.7% and OS rate of 69.2%.
Since most PBT data from early-to-intermediate-stage HCC were obtained from patients with recurrent or residual HCC, the recently developed 2022 Korean Liver Cancer Association-National Cancer Center Korea practice guidelines for the management of HCC described the role of EBRT including PBT as an effective local treatment option limited to small recurrent HCC [55]. However, PBT has demonstrated similar efficacy in terms of local control, survival, and toxicity in treatment-naïve HCC patients compared to those with recurrent HCC, as the data mentioned above. Hence, we may have to assume that PBT has a potential role as a first-line therapy for treatment-naïve HCC patients as well as those with recurrent or residual HCC. Overall, the data suggest that PBT could be an effective alternative or complementary local treatment for early-to-intermediate-stage HCC where other local treatments, such as surgical resection, ablative therapy, and TACE, might be unsuitable or ineffective, and could be a potential first-line treatment in treatment-naïve HCC patients. In addition, PBT was an effective way to increase the chances of curing localized HCC in liver transplantation candidates as definitive or bridging therapy while they wait for transplantation with less morbidity compared to TACE [52].
Advanced-stage HCC (BCLC stage C)
The recommended treatment for advanced-stage HCC, BCLC C, is systemic therapy, such as sorafenib, with an expected median survival time of about 10 months. A recent randomized trial comparing atezolizumab plus bevacizumab to sorafenib showed that the combination of atezolizumab and bevacizumab significantly improved objective responses and OS compared to sorafenib [56]. However, the objective response was still around 20–30%, and relatively selected patients, such as only those in the Child-Pugh A class, participated and more than 50% of the patients experienced serious adverse events (grade 3–4 toxicity) [56].
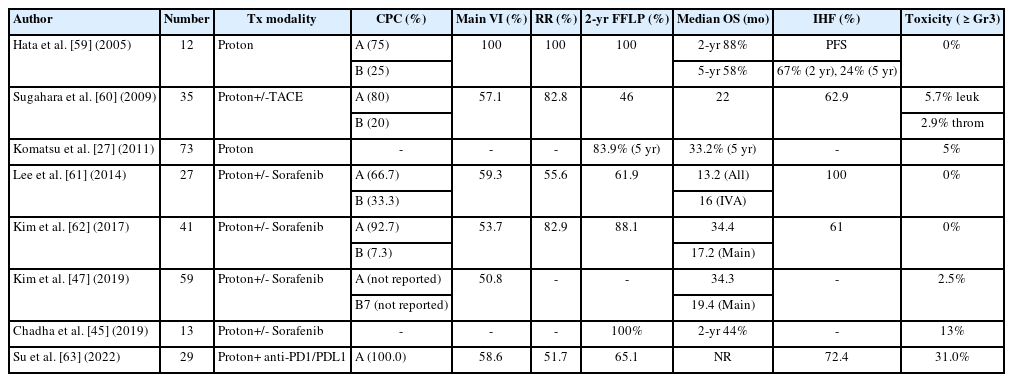
Nevertheless, local treatments, such as PBT, still may have a role in advanced HCC, especially in HCC with vascular invasion. A randomized controlled study compared combination of EBRT plus TACE with sorafenib in HCC with macroscopic vascular invasion, and demonstrated superior efficacy of EBRT plus TACE with survival improvement compared to systemic therapy [57]. In neoadjuvant setting, EBRT provided significantly better postoperative survival outcomes in patients with resectable HCC and PVTT [58]. Table 2 summarizes the results of previously published PBT data for HCC patients with vascular invasion. The reported median survival time of those patients after PBT was 16 to 22 months [27,45,47,59-63]. These PBT outcomes were better than the outcomes expected of systemic treatment. In particular, the response rate was remarkably high, at up to 60–100%. After PBT, recanalization of the portal vein followed by the restoration of liver function might be a possible reason for the improvement in survival. In addition, treatment-related toxicity was relatively mild compared to systemic treatment, with ≥grade 3 toxicity rates of 0–13% after PBT. Among EBRT modalities for the treatment of HCC with macrovascular invasion, a recent meta-analysis showed a significantly better OS rate in the PBT group, which was 61%, 45%, and 45% in the PBT, conventional EBRT, and SBRT groups, respectively (P<0.05 for each comparison) [64]. For future perspectives, the combination of PBT with immune-checkpoint inhibitors such as anti-PD1/PDL1 is thought to be a promising treatment option, showing improved progression-free survival up to 27 months with curative intent treatment in a single retrospective study [63].
CONCLUSION
In conclusion, since PBT has a dosimetric benefit through superior physical properties, it has been thought to have advantages over conventional EBRT, as well as other local therapies, in the management of patients with HCC. Numerous clinical studies have demonstrated PBT as a highly effective local therapeutic option for early-to-advanced HCC, with favorable survival outcomes and a low toxicity rate. PBT is also being used successfully in challenging clinical conditions, such as major vascular invasion and re-irradiation cases. Nevertheless, the clinical evidence for PBT in HCC has been considered insufficient so far, as most studies may have involved inherent selection biases of a retrospective nature and biases towards new technologies, and it contains relatively few patients for high-level evidence. Hence, further research is warranted, and some studies are currently underway, including comparisons of PBT with other treatment modalities or combinations, as well as other types of EBRT, such as photon EBRT or CIRT. For instance, comparisons of PBT versus ablative therapy in patients with treatment-naïve early-stage HCC, the efficacy of combinations of PBT and TACE or SIRT in intermediate-stage disease, or combinations of PBT and systemic therapy in advanced-stage disease will be promising research topics for future clinical trials and will enhance evidence-based clinical guidance and improve patient selection.
Notes
Authors’ contribution
Conception, design of the study, literature review and analysis, critical revision and editing, and final approval of the final version: SU Lee and TH Kim.
Conflicts of Interest
The authors have no conflicts to disclose.
Acknowledgements
This study supported by National Cancer Center grant (NCC-2110351).
Abbreviations
HCC
hepatocellular carcinoma
TACE
transarterial chemoembolization
SIRT
selective internal radiotherapy
EBRT
external beam radiation therapy
IMRT
intensity modulated radiation therapy
VMAT
volumetric-modulated arc therapy
SBRT
stereotactic body radiation therapy
PBT
proton beam therapy
CIRT
carbon ion therapy
OAR
organ at risk
RILD
radiation-induced liver disease
SOBP
spread-out Bragg peak