| Clin Mol Hepatol > Volume 29(4); 2023 > Article |
|
ABSTRACT
Steatotic liver disease was suggested as an overarching term encompassing various etiologies of hepatic steatosis. Experts from multinational liver societies went through the Delphi process, including four rounds of surveys, and consented to adopt a new nomenclature and definition instead of the conventional nonalcoholic fatty liver disease (NAFLD). This was to improve the understanding of the patients and primary care physicians, with an explanation of the pathophysiology in the name of the disease. Also, it could minimize the stigmatization of patients by using the histological neutral term “steatosis” instead of “fatty”. Herein, we will discuss the changes and continuity between the two nomenclatures, metabolic dysfunction-associated steatotic liver disease (MASLD) and NAFLD, as well as the challenges to MASLD which need to be addressed in future.
In the 2023 European Association for the Study of the Liver Congress, ‘steatotic liver disease (SLD)’ was suggested as an overarching term encompassing various etiologies of hepatic steatosis. Experts from multinational liver societies went through the Delphi process about the possible changes, candidates for the nomenclature, impact on the routine clinical practice, etc. They proposed a new nomenclature and definition instead of the conventional “nonalcoholic fatty liver disease” (NAFLD). This was to improve the understanding of disease to patients and primary care physicians (PCPs). Also, it could minimize the stigmatization of patients by using the histological neutral term “steatosis” instead of “fatty” [1].
SLD is diagnosed either histologically or by imaging. SLD is further divided into two sub-categories: SLD with cardiometabolic risk factors (CMRF) and SLD without CMRF (Fig. 1). The former is named ‘metabolic dysfunction-associated steatotic liver disease (MASLD)’ when no other etiologies coexist. Metabolic dysfunction is defined as having one or more CMRF, including a body mass index of ≥25; waist circumference of ≥94 cm in Western men and ≥80 cm in Western women; presence of impaired fasting glucose, impaired glucose tolerance, or diabetes mellitus; high blood pressure, high plasma triglyceride levels; lower plasma high-density lipoprotein levels; or dyslipidemia (Table 1). As the consensus was proposed from the Western societies, the cut-offs for waist circumference of Eastern men and women were not clearly provided.Referring to the cut-offs provided in the metabolic dysfunction-associated fatty liver disease (MAFLD) consensus from the Asian Pacific Association for the Study of the Liver [2], the cut-offs would be ≥90 cm in Asian men and ≥80 cm in Asian women.
Herein, we will discuss the changes and continuity between the two nomenclatures, MASLD and NAFLD, as well as the challenges to MASLD which need to be addressed in future.
In NAFLD, the exclusion of various factors for hepatic steatosis (i.e., alcohol, viruses, and drugs) is the initial major step in diagnosis. Patients with viral hepatitis and hepatic steatosis with nonalcoholic or metabolic causes are diagnosed with viral hepatitis plus fatty liver but not NAFLD. Owing to the absence of approved drugs for NAFLD and difficulty in lifestyle modifications, the presence of fatty liver has been often overlooked [3]. The impact of hepatic steatosis on the long-term outcomes of viral hepatitis is complicated. For example, hepatic steatosis may lead to a higher chance of hepatitis B surface antigen seroclearance [4]. However, concurrent hepatic steatosis may exacerbate liver fibrosis [5], increase the risk of hepatocellular carcinoma (HCC) and is associated with an increased overall mortality [6]. Additionally, an increased number of CMRFs is associated with an increased risk of overall mortality, HCC, and extrahepatic cancers [7]. Therefore, increasing the awareness of both the patients and PCPs for cardiometabolic risks, which may coexist with other liver diseases, is crucial. In this regard, the new term MASLD has enabled us to characterize the multiple etiologies of liver disease and to treat patients holistically by ruling in metabolic dysfunction as the underlying pathophysiology without ruling out alcohol. This allows patients to identify and manage health issues across multiple dimensions. In the presence of other etiologies of steatosis, including significant alcohol intake, it is subclassified as SLD with other etiologies. Alcohol is a major combination etiology of SLD, in addition to CMRFs. Significant alcohol intake was quantified as a weekly intake of 140 g or more in women and 210 g or more in men. Therefore, they named it metabolic dysfunction-associated steatotic liver disease with greater alcohol consumption (MetALD) as the representative of this subclass, which signifies the presence of CMRFs and significant alcohol intake as the dual etiologies of hepatic steatosis. However, this subclass is not confined to MetALD; other combination etiologies of steatosis with CMRFs can be included (Fig. 1).
Overall, the adoption of the overarching term, SLD, offers the advantages of comprehensive patient management and a more holistic understanding of liver diseases.
It is difficult for patients to understand the pathophysiology and treatment options for NAFLD, as the name only implies that it is not related to alcohol intake. However, with the introduction of the new concepts of MASLD, MetALD, and alcohol-related liver disease (ALD), it could deliver more intuitive messages to patients and PCPs about disease etiology and the direction of future treatment. In particular, MASLD emphasizes metabolic dysfunction as its pathogenesis and conveys a direct message to patients that metabolic parameters should be managed for effective treatment of the disease. In summary, the positive criteria for the diagnosis of MASLD provide patients and PCPs with a more patient-friendly and comprehensible way of communicating about the disease.
In English-speaking countries, the term “fatty” is often associated with negative connotations, leading to social stigma and patient discomfort. In one study that surveyed 144 patients with NAFLD in a liver clinic located in Spain, 69% of them responded that stigma was perceived to affect all four domains: stereotypes, discrimination, shame, and social isolation [8]. In another global survey of patients and healthcare providers, 25–31% of the patients felt uncomfortable about the diagnostic terms of NAFLD, while 32–49% of the healthcare providers felt the term was stigmatizing to the patients [9]. Patients would suffer negative stereotypes from the word “Fatty” in that they are perceived to be lazy, unmotivated, and lacking in their willpower to control their self-inflicted disease [10].
To address this issue and prevent unintended social stigma, the new nomenclature task force team has recommended using the histologic term “steatosis,” instead of “fatty” in the diagnosis [1]. A Delphi process was conducted with 236 liver disease professional panelists from 56 countries. The results revealed that 61% and 66% of respondents considered the terms “non-alcohol” and “fatty” to be stigmatizing. Furthermore, 74% of the respondents believed that these terms were significantly flawed and advocated for renaming the condition. On the other hand, the perceptions of stigma from the providers to the patients may differ according to different languages and cultures, for example, 32% from East Asia vs. 49% in the United States [9]. In some regions, there might be no social stigma associated with the terms “fatty” or “nonalcoholic,” and patients may feel comfortable with these labels. Additionally, there could be areas where it is challenging to effectively differentiate between the term “fatty” and the newly proposed term “steatosis” due to unique language characteristics. Depending on the regions and cultural contexts, the transition from “fatty” to “steatosis” may either be ambiguous or not possible. Additionally, there is an issue of over-medicalizing the term as steatosis is confusing to patients [1,10]. However, this trial aimed to foster a more supportive and understandable environment for patients by minimizing stigmatization, encouraging better communication and care in managing the disease.
In real-world scenarios, distinguishing between MASLD and MetALD is intricate and challenging due to the complex interplay of alcohol consumption and metabolic risk factors. Moreover, the classification of MASLD, MetALD, and ALD often faces inaccuracies, as it relies on self-reported alcohol intake data, which can underestimate the actual alcohol consumption in many cases. Despite these challenges, alcohol and metabolic risk factors collectively contribute to an escalated risk of severe liver disease. While cardiovascular disease emerges as the leading cause of death in MASLD, leading cause of death of MetALD and ALD is liver-related mortality [11]. The new nomenclature of SLD offers coexistence of alcohol use and metabolic risk factors, recognizing the conditions as part of a disease spectrum rather than exclusive entities. MetALD is a category with a continuum across MASLD and ALD depending on the amount of alcohol consumption.
During the transition of the new nomenclature from the previous NAFLD, several sensitive issues should be addressed. 3 One critical concern is whether previous epidemiologic data and diagnostic cut-offs of NITs can still be applied under the new diagnostic criteria. Additionally, the impact of this new nomenclature on ongoing clinical trials for NAFLD and related drug development is of significant importance. Radical changes in the diagnostic criteria may lead to the loss of valuable epidemiological data that have accumulated over the decades. It will also necessitate the collection of new epidemiological data as well as data on the disease’s clinical course and long-term outcomes under the new nomenclature. This can further necessitate the re-evaluation of the diagnostic performance of various NITs [12], and the cost-effectiveness of screening strategies based on new disease transition rates. To address these concerns, the nomenclature task force team analyzed data from the LITMUS cohort. Notably, 98% of the NAFLD cohort fulfill the new criteria for MASLD. And very recently proportion of overlap between NAFLD and MASLD was somewhat different from Asian community cohort in Hongkong (97.7%) [13]. This suggests that MASLD can take over data of epidemiology, NITs, and clinical trials from the previous NAFLD era.
The new SLD classification underscores the significance of alcohol consumption by categorizing it into MASLD, MetALD, and ALD based on amount of alcohol intake. Numerous research studies have highlighted the correlation between alcohol consumption and heightened risks of liver fibrosis and HCC among individuals with NAFLD, with the extent of risk associated with the quantity of alcohol consumed [11]. This classification system places ongoing emphasis on both alcohol intake and the management of metabolic factors, by delineating the disease spectrum according to alcohol intake levels.
SLD is an umbrella term encompassing various conditions, including MASLD, MetALD, ALD, single-specific etiology SLD, and cryptogenic SLD. MASLD is expected to overlap with previous NAFLD patients in approximately 97–98%. This suggests that the NIT and its cut-off applied in previous NAFLD patients can be used without significant changes [14]. However, it is unclear whether previous NITs can be effectively applied in MetALD. Moreover, given the poor diagnostic performance of NITs in patients with alcoholic liver disease [15], there is a need to develop new NITs tailored to ALD. Each SLD subgroup exhibits distinct characteristics and varying degrees of advanced liver fibrosis, which may necessitate different NITs and their respective cutoff values for an accurate diagnosis. Therefore, validating the diagnostic performance of NITs in SLD subgroups and developing individualized screening algorithms for high-risk groups are needed in these subcategories.
In the recent proposal of the MASLD consensus, SLD without CMRFs is further classified by the criteria of either the presence or absence of a specific etiology of hepatic steatosis: SLD with other specific etiologies or cryptogenic SLD (i.e., SLD without other specific etiologies of hepatic steatosis). Indeed, metabolic dysfunction and alcohol consumption are the major causes of SLD. However, intestinal dysbiosis, genetic variants (e.g., PNPLA3, TM6SF2, etc.), and sarcopenia may also contribute to the development of SLD [16,17], especially in lean or non-obese individuals [18,19]. In the current SLD classification system, lean or non-obese NAFLD may be divided into MASLD and cryptogenic SLD according to the presence of CMRFs, regardless of the genetic variants, intestinal dysbiosis, and sarcopenia. Overemphasis on the CMRFs may overlook or underestimate the involvement of these various disease modifiers, similar to the drawbacks originating from the rule-out diagnosis of NAFLD. In addition, nutrition associated SLD should not be overlooked and it would be further categorized in the cryptogenic SLD. This would also lead to a comprehensive understanding of the contributing factors and improve patient management strategies.
MAFLD critically addressed various issues relating negative diagnostic criteria and provided valuable insights for NAFLD. Moreover, MAFLD introduced a concept that offers a more intuitive and fundamental approach by considering the social stigma caused by nomenclature. However, including patients with significant alcoholic liver disease and viral hepatitis in the MAFLD group resulted in increased heterogeneity of the target population based on clinical characteristics and prognosis. This heterogeneity can pose a significant limitation in the development of future disease screening strategies and drug development approaches. In contrast, MASLD effectively resolves the heterogeneity concerns raised by MAFLD, particularly those related to the inclusion of patients with significant alcoholic liver disease and viral hepatitis, while acknowledging the fundamental problems highlighted by MAFLD. Furthermore, the newly introduced MASLD classification encompasses almost the entire previous NAFLD patient population (>95%), enabling a seamless continuation of ongoing clinical trials for NAFLD drugs and successful incorporation of diagnostic tool data. In conclusion, the newly proposed MASLD effectively subdivides the SLD subgroups based on various etiological causes while embracing the important foundational issues presented by the previous MAFLD. This approach maximized the homogeneity of each subgroup, offering a promising pathway for targeted disease screening strategies and drug development.
Indeed, the transition to a new nomenclature for the SLD requires a step-by-step approach to ensure its successful implementation. First, we need more discussions with various stakeholders, including researchers, authorities, and patient groups, regarding clinical trials. We also need to cooperate with liver societies and organizations that have leadership in the Asia-Pacific region. In particular, the Asian Pacific Association for the Study of the Liver officially still supports MAFLD and casts doubt on MASLD. Second, strategies are needed to enhance patient awareness and educate PCPs by disseminating educational materials. It is expected that most patients with SLD will not be managed by hepatologists or gastroenterologists in a referral center, but rather by PCPs. A well-coordinated and comprehensive approach involving all relevant stakeholders will facilitate a successful transition to a new nomenclature for SLD and improve the diagnosis, management, and outcomes of patients with this complex liver disease.
ACKNOWLEDGMENTS
This work was partly supported by the Korea Drug Development Fund funded by the Ministry of Science and ICT, Ministry of Trade, Industry, and Energy, and Ministry of Health and Welfare HN21C1149000023, Republic of Korea.
This research was supported by a grant of Patient-Centered Clinical Research Coordinating Center (PACEN) funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HC23C0058).
Figure 1.
The classification of Steatotic liver disease. Steatotic liver disease is diagnosed based on the presence of hepatic steatosis identified by imaging or liver biopsy. Metabolic dysfunction-associated steatotic liver disease or metabolic dysfunction-associated alcohol-related liver disease or other combination etiologies are diagnosed with the presence of cardiometabolic risk factors. Steatosis without cardiometabolic risk factors is further sub-classified into SLD with other specific etiology of hepatic steatosis or cryptogenic steatotic liver disease. Figure is modified from the recent consensus proposal [1]. MetALD, metabolic dysfunction-associated alcohol-related liver disease; SLD, steatotic liver disease.
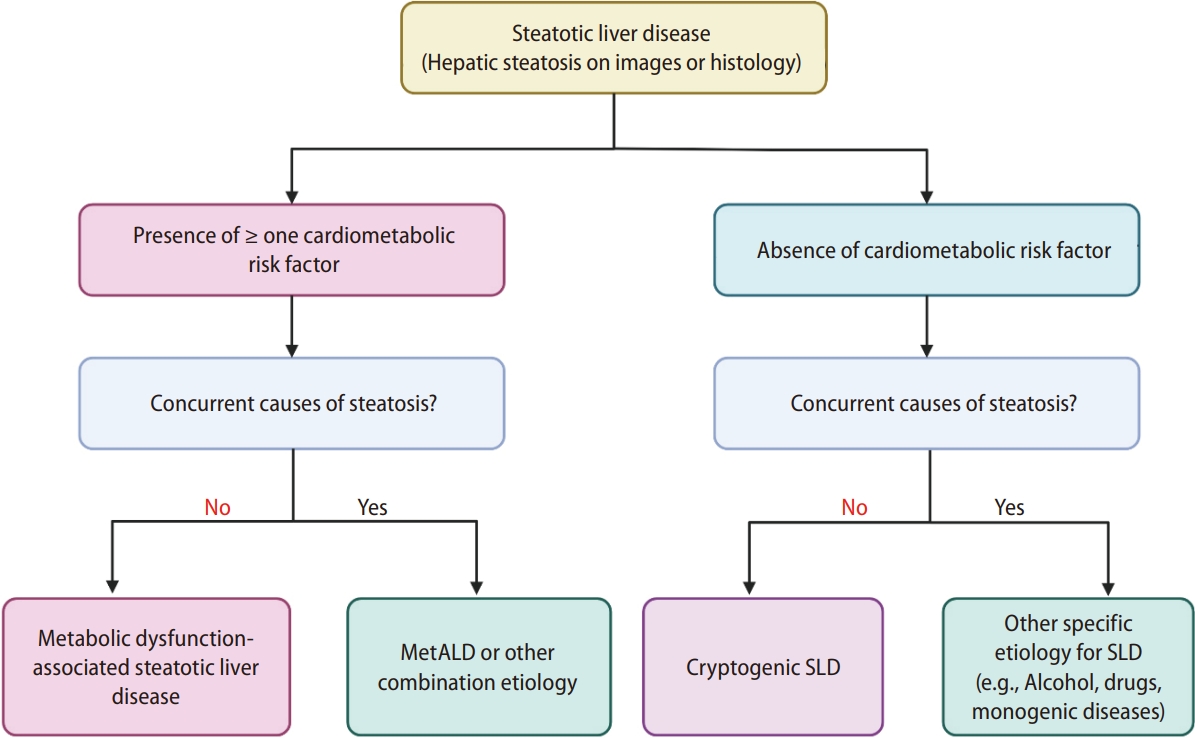
Table 1.
The criteria for cardiometabolic risk factors for adults
Abbreviations
ALD
alcohol-related liver disease
CMRF
cardiometabolic risk factor
MAFLD
metabolic dysfunction-associated fatty liver disease
MASLD
metabolic dysfunctionassociated steatotic liver disease
MetALD
metabolic dysfunction-associated
NAFLD
nonalcoholic fatty liver disease
NIT
non-invasive test
PCP
primary care physicians
REFERENCES
1. Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol 2023 Jun 20. doi: 10.1016/j.jhep.2023.06.003.


2. Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 2020;158:1999-2014.e1.


3. Yoon EL, Jun DW. Changing the nomenclature from nonalcoholic fatty liver disease to metabolic dysfunction-associated fatty liver disease is more than a change in terminology. Clin Mol Hepatol 2023;29:371-373.




4. Wong YJ, Nguyen VH, Yang HI, Li J, Le MH, Wu WJ, et al. Impact of fatty liver on long-term outcomes in chronic hepatitis B: a systematic review and matched analysis of individual patient data meta-analysis. Clin Mol Hepatol 2023;29:705-720.




5. Yang M, Wei L. Impact of NAFLD on the outcome of patients with chronic hepatitis B in Asia. Liver Int 2022;42:1981-1990.

6. Kim MN, Han K, Yoo J, Hwang SG, Ahn SH. Increased risk of hepatocellular carcinoma and mortality in chronic viral hepatitis with concurrent fatty liver. Aliment Pharmacol Ther 2022;55:97-107.



7. Lee YB, Moon H, Lee JH, Cho EJ, Yu SJ, Kim YJ, et al. Association of metabolic risk factors with risks of cancer and all-cause mortality in patients with chronic hepatitis B. Hepatology 2021;73:2266-2277.



8. Carol M, Pérez-Guasch M, Solà E, Cervera M, Martínez S, Juanola A, et al. Stigmatization is common in patients with non-alcoholic fatty liver disease and correlates with quality of life. PLoS One 2022;17:e0265153.



9. Younossi Z, Yılmaz Y, Fan JG, Wong VWS, El Kassas M, Zelber-Sagi S, et al. Stigma in NAFLD and NASH: a global survey of patients and providers. J Hepatol 2023;78:S627-S628.

10. Shiha G, Korenjak M, Casanovas T, Mooney V, Sigurðardóttir S, Koulla Y, et al. MAFLD 2022: An ELPA/ALPA/EASO-ECPO joint statement on disease stigma. J Hepatol 2022;77:1717-1719.


11. Karlsen TH, Sheron N, Zelber-Sagi S, Carrieri P, Dusheiko G, Bugianesi E, et al. The EASL-Lancet Liver Commission: protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet 2022;399:61-116.


12. Park H, Yoon EL, Kim M, Lee J, Kim JH, Cho S, et al. Comparison of diagnostic performance between FIB-4 and NFS in metabolic-associated fatty liver disease era. Hepatol Res 2022;52:247-254.



13. Song SJ, Che-To Lai J, Lai-Hung Wong G, Wai-Sun Wong V, Cheuk-Fung Yip T. Can we use old NAFLD data under the new MASLD definition? J Hepatol 2023 Aug 2. doi: 10.1016/j.jhep.2023.07.021.


14. Zhang S, Mak LY, Yuen MF, Seto WK. Screening strategy for non-alcoholic fatty liver disease. Clin Mol Hepatol 2023;29(Suppl):S103-S122.




15. Graupera I, Thiele M, Serra-Burriel M, Caballeria L, Roulot D, Wong GL, et al. Low accuracy of FIB-4 and NAFLD fibrosis scores for screening for liver fibrosis in the population. Clin Gastroenterol Hepatol 2022;20:2567-2576 e6.


16. Sookoian S, Pirola CJ. Genetics in non-alcoholic fatty liver disease: The role of risk alleles through the lens of immune response. Clin Mol Hepatol 2023;29(Suppl):S184-S195.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





