| Clin Mol Hepatol > Volume 30(2); 2024 > Article |
|
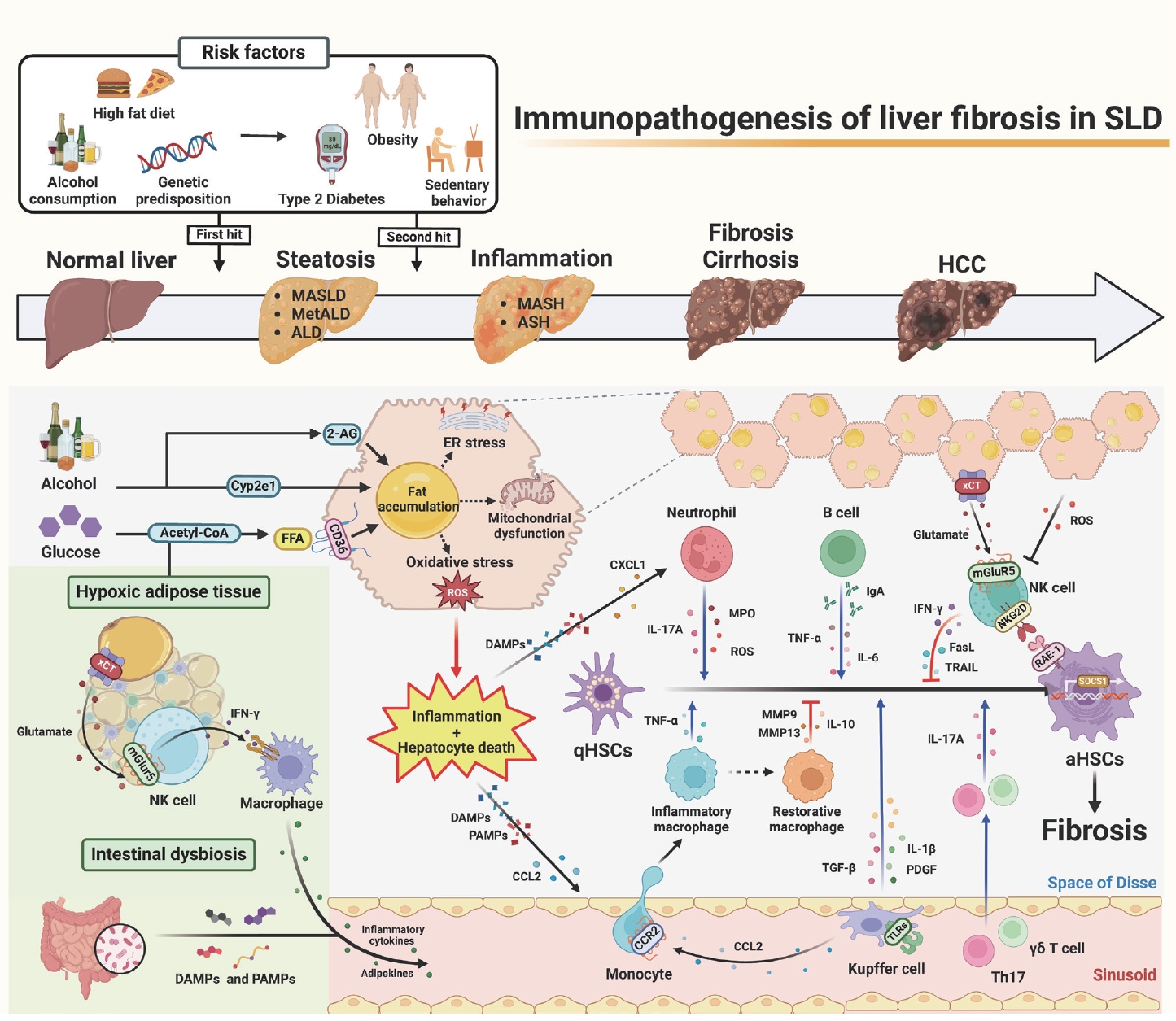
Recently, a new term, steatotic liver disease (SLD), has been introduced to the field of hepatology to encompass the various etiologies of steatosis [1,2]. Hepatic steatosis, having an excessive fat accumulation, has been considered a key factor in the progression of serious liver diseases such as hepatic inflammation, fibrosis/cirrhosis, and hepatocellular carcinoma [2-4]. It could be initiated by an overload of nutrition (e.g., glucose and free fatty acids), intoxicants (e.g., alcohol and endocannabinoid-mediated metabolic dysfunction), or genetic factors [3-5]. Interestingly, lines of evidence have suggested that beyond its role as a metabolic organ, the liver is considered an immunological and neurological organ due to its diverse metabolic functions, enriched immune cells (e.g., innate and adaptive immune cells), and production and release of hepatotransmitters such as glutamate or 2-arachidonoylglycerol (2-AG) [4-6]. So, these factors might affect the progression of SLD, from simple steatosis to steatohepatitis and fibrosis, by regulating the activation of hepatic stellate cells (HSCs).
It has been reported that inflammation and subsequent liver fibrosis occur due to various immune-mediated activations of HSCs through eating habit-mediated metabolic dysfunction (e.g., obesity, type 2 diabetes, or chronic alcohol consumption), sedentary behavior, and genetic predisposition [4,5]. Steatohepatitis may be triggered by prolonged multiple stresses in fat-storing hepatocytes, such as endoplasmic reticulum (ER) stress, mitochondria dysfunction, and oxidative stress, including alcohol-induced reactive oxygen species (ROS) and lipotoxicity [5,6]. These cellular stresses, combined with diverse harmful factors, such as inflammatory cytokines, adipokines, short-chain fatty acids, and pathogen- or damage-associated molecular patterns (PAMPs or DAMPs) originated from adipose tissue (AT) and the intestine, may cause hepatocyte death and inflammation [7]. Recently, an interesting study suggested glutamate-mediated cross-talk between AT and liver, in which hypoxic adipocytes excreted glutamate through the xCT transporter and the resulting production of interferon-╬│ (IFN-╬│) in natural killer (NK) cells by metabotropic glutamate receptor 5 (mGluR5), subsequently leading to the augmentation of adipose macrophage activation and steatohepatitis in obese mice and patients [8].
Through these cellular responses and organ cross-talks, inflammatory immune cells migrate nearby damaged hepatocytes and stimulate the transformation of quiescent HSCs (qHSCs) into activated HSCs (aHSCs) by producing various mediators, including cytokines, chemokines, and extracellular vesicles. Injured fat-storing hepatocytes produce chemokines, such as C-C motif chemokine 2 (CCL2) and CXC motif chemokine ligand 1 (CXCL1), to recruit pro-inflammatory macrophages and neutrophils, respectively. After migration, macrophages, neutrophils, and resident Kupffer cells are stimulated by DAMPs (e.g., ATP, mitochondrial DNA, or double-stranded RNA), PAMPs (e.g., LPS, CpG DNA, Flagellin), or cytokines through specific receptors, including P2X purinoceptor 7 and toll-like receptors (e.g., TLR2, TLR3, TLR4, TLR5, and TLR9) [7]. Then, the production of tumor necrosis factor (TNF)-╬▒, interleukin (IL)-1╬▓, platelet derived growth factor (PDGF), IL-17A, myeloperoxidase (MPO), transforming growth factor (TGF)-╬▓1, and ROS by these cells leads to the activation of qHSCs [9,10]. Moreover, several types of lymphocytes, such as Th17 cells, ╬│╬┤T cells, and B cells, augment HSC activation by producing IL-17, TNF-╬▒, and IL-6 [9,11,12].
In contrast, several types of cells, such as NK cells and restorative macrophages, can kill aHSCs or suppress HSC activation by producing death ligands, including TNF-related apoptosis-inducing ligand (TRAIL) and Fas ligand (FasL), IFN-╬│, anti-inflammatory IL-10, and matrix metalloproteinases (MMPs) [9,13]. Once activated HSCs express retinoic acid early inducible 1 (RAE-1), a specific ligand for NKG2D, NK cells specifically produce IFN-╬│, TRAIL, or FASL through the NKG2D-RAE-1 interaction [13]. In addition, Ly6Clow F4/80+ CD11b+ macrophages can induce fibrosis resolution by producing MMPs or Gr1+ CD11b+ bone marrow cells, which inhibit inflammation by IL-10 production in the early stage of liver fibrosis [9,14]. However, prolonged injuries (e.g., chronic alcohol consumption) cause HSCs to have the ability to avoid this cytotoxicity by increasing TGF-╬▓ production and suppressor of cell signaling 1 (SOCS1) expression in aHSCs [5,15]. Moreover, ROS might deplete NK subpopulations or suppress NK cytotoxicity in advanced liver fibrosis [16,17]. A recent study suggested an interesting solution for how to overcome HSC evasion against NK cytotoxicity, in which mGluR5 activation in NK cells by hepatic glutamate, a hepatotransmitter, further enhanced NK cytotoxicity to aHSCs, resulting in the attenuation of liver fibrosis [17]. Accordingly, the delivery of mGluR5-stimulated NK cells through the tail vein improved liver fibrosis in mice [17], suggesting a therapeutic intervention of liver fibrosis in patients.
In the past, SLD was somewhat neglected, but as viral hepatitis has recently been overcome, the possibility of SLD progression to serious chronic liver disease has emerged. Despite pro-inflammatory immune responses for SLD progression to liver fibrosis, it is noteworthy that certain immune cells (e.g., NK cells or restorative macrophages) may have strong anti-fibrotic functions. In particular, new therapeutics can be developed by carefully looking at intrahepatic neurological signaling (e.g., 2-AG production in HSCs and mGluR5 activation in NK cells). Therefore, precise molecular pathways related to the immunopathogenesis of liver fibrosis should be extensively investigated. Notably, further research will be needed to determine how the neuro-metabo-immune axis, still minimally investigated, influences SLD and liver fibrosis.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea grant funded by the Ministry of Science and ICT (2021R1A2C3004589, 2022M3A9B6017654, RS-2023-00223831).
FOOTNOTES
Abbreviations
SLD
steatotic liver disease
2-AG
2-arachidonoylglycerol
HSC
hepatic stellate cell
ER
endoplasmic reticulum
ROS
reactive oxygen species
PAMP or DAMP
pathogenor damage-associated molecular pattern
AT
adipose tissue
IFN-╬│
interferon-╬│
NK
natural killer
mGluR5
metabotropic glutamate receptor 5
qHSC
quiescent HSCs
aHSC
activated HSCs
CCL2
C-C motif chemokine 2
CXCL1
CXC motif chemokine ligand 1
TLR
toll-like receptor
TNF
tumor necrosis factor
IL
interleukin
PDGF
platelet-derived growth factor
MPO
myeloperoxidase
TGF
transforming growth factor
TRAIL
TNF-related apoptosis-inducing ligand
FasL
Fas ligand
MMP
metalloproteinase
RAE-1
retinoic acid early inducible 1
SOCS1
suppressor of cell signaling 1
REFERENCES
1. Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol 2023;79:1542-1556.

2. Kim GA, Moon JH, Kim W. Critical appraisal of metabolic dysfunction-associated steatotic liver disease: Implication of Janusfaced modernity. Clin Mol Hepatol 2023;29:831-843.




3. Lee KC, Wu PS, Lin HC. Pathogenesis and treatment of nonalcoholic steatohepatitis and its fibrosis. Clin Mol Hepatol 2023;29:77-98.




4. Huby T, Gautier EL. Immune cell-mediated features of nonalcoholic steatohepatitis. Nat Rev Immunol 2022;22:429-443.




5. Seitz HK, Bataller R, Cortez-Pinto H, Gao B, Gual A, Lackner C, et al. Alcoholic liver disease. Nat Rev Dis Primers 2018;4:16.



6. Choi WM, Kim HH, Kim MH, Cinar R, Yi HS, Eun HS, et al. Glutamate signaling in hepatic stellate cells drives alcoholic steatosis. Cell Metab 2019;30:877-889 e7.



7. Shim YR, Jeong WI. Recent advances of sterile inflammation and inter-organ cross-talk in alcoholic liver disease. Exp Mol Med 2020;52:772-780.




8. Kim HH, Shim YR, Kim HN, Yang K, Ryu T, Kim K, et al. xCTmediated glutamate excretion in white adipocytes stimulates interferon-╬│ production by natural killer cells in obesity. Cell Rep 2023;42:112636.


9. Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol 2017;14:397-411.



10. Zhou Z, Xu MJ, Cai Y, Wang W, Jiang JX, Varga ZV, et al. Neutrophil-hepatic stellate cell interactions promote fibrosis in experimental steatohepatitis. Cell Mol Gastroenterol Hepatol 2018;5:399-413.



11. Thapa M, Chinnadurai R, Velazquez VM, Tedesco D, Elrod E, Han JH, et al. Liver fibrosis occurs through dysregulation of MyD88- dependent innate B-cell activity. Hepatology 2015;61:2067-2079.




12. Lee JH, Shim YR, Seo W, Kim MH, Choi WM, Kim HH, et al. Mitochondrial double-stranded RNA in exosome promotes interleukin-17 production through toll-like receptor 3 in alcohol-associated liver injury. Hepatology 2020;72:609-625.




13. Jeong WI, Park O, Radaeva S, Gao B. STAT1 inhibits liver fibrosis in mice by inhibiting stellate cell proliferation and stimulating NK cell cytotoxicity. Hepatology 2006;44:1441-1451.


14. Suh YG, Kim JK, Byun JS, Yi HS, Lee YS, Eun HS, et al. CD11b(+) Gr1(+) bone marrow cells ameliorate liver fibrosis by producing interleukin-10 in mice. Hepatology 2012;56:1902-1912.



15. Jeong WI, Park O, Suh YG, Byun JS, Park SY, Choi E, et al. Suppression of innate immunity (natural killer cell/interferon-╬│) in the advanced stages of liver fibrosis in mice. Hepatology 2011;53:1342-1351.



-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,943 View
- 80 Download
- ORCID iDs
-
Won-Il Jeong

https://orcid.org/0000-0002-4240-4631 - Related articles
-
Implications of comorbidities in nonalcoholic fatty liver disease2023 April;29(2)
Non-invasive biomarkers of liver fibrosis in non-alcoholic fatty liver disease2023 April;29(2)
Identification of high-risk subjects in nonalcoholic fatty liver disease2023 February;29(Suppl)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



