| Clin Mol Hepatol > Volume 29(4); 2023 > Article |
|
ABSTRACT
Background/Aims
Understanding of non-alcoholic fatty liver disease (NAFLD) continues to expand, but the relationship between race and ethnicity and NAFLD outside the use of cross-sectional data is lacking. Using longitudinal data, we investigated the role of race and ethnicity in adverse outcomes in NAFLD patients.
Methods
Patients with NAFLD confirmed by imaging via manual chart review from any clinics at Stanford University Medical Center (1995ŌĆō2021) were included. Primary study outcomes were incidence of liver events and mortality (overall and non-liver related).
Results
The study included 9,340 NAFLD patients: White (44.1%), Black (2.29%), Hispanic (27.9%), and Asian (25.7%) patients. For liver events, the cumulative 5-year incidence was highest among White (19.1%) patients, lowest among Black (7.9%) patients, and similar among Asian and Hispanic patients (~15%). The 5-year and 10-year cumulative overall mortality was highest for Black patients (9.2% and 15.0%, respectively, vs. 2.5ŌĆō3.5% and 4.3ŌĆō7.3% in other groups) as well as for non-liver mortality. On multivariable regression analysis, compared to White patients, only Asian group was associated with lower liver-related outcomes (aHR: 0.83, P=0.027), while Black patients were at more than two times higher risk of both non-liver related (aHR: 2.35, P=0.010) and overall mortality (aHR: 2.13, P=0.022) as well as Hispanic patients (overall mortality: aHR: 1.44, P=0.022).
Conclusions
Compared to White patients, Black patients with NAFLD were at the highest risk for overall and non-liver-related mortality, followed by Hispanic patients with Asian patients at the lowest risk for all adverse outcomes. Culturally sensitive and appropriate programs may be needed for more successful interventions.
Graphical Abstract
Nonalcoholic fatty liver disease (NAFLD) is a chronic liver disease in which steatosis is present in greater than 5% of the liver cells. NAFLD is a progressive disease in up to 20% of patients, but until recently, there has been a paucity of non-invasive tests for both steatosis and fibrosis diagnoses, so our understanding of progressive NAFLD has mostly come from those who have undergone a liver biopsy or abdominal imaging which limits large population studies [1-16].
As such, our understanding of the factors associated with adverse outcomes of NAFLD, such as race and ethnicity, is evolving. From prior studies conducted in the United States, Hispanic origin is associated with the highest risk of having NAFLD, while those of non-Hispanic Black origin have a lower risk of having NAFLD but a higher risk for adverse outcomes, including mortality [17-23]. However, the majority of prior studies on race, ethnicity, and NAFLD used data from large population-based databases that preclude survival analysis or were limited by the availability of follow-up outcomes.
Therefore, the purpose of this study was to use individual patient-level data from a large medical center to provide a longitudinal picture of the role of ethnicity in patients residing in the United States who have NAFLD.
We retrospectively identified patients with NAFLD at Stanford University Medical Center, Palo Alto, California, USA, between 1995 and 2021. NAFLD was confirmed by the presence of hepatic steatosis in abdominal ultrasound, computed tomography, or magnetic resonance imaging on manual chart review. We excluded patients with significant alcohol use and/or concurrent viral hepatitis, autoimmune hepatitis, alpha-1 antitrypsin deficiency, hemochromatosis, or WilsonŌĆÖs disease. Data on race and ethnicity are extracted from the demographics section in the electronic medical records, which are self-reported by the patients. Patients with unknown, mixed race and ethnicity, or race and ethnicity other than White, Black, Hispanic, or Asian, were excluded due to small numbers. The final study cohort was grouped into four race and ethnicity groups: White, Black, Hispanic, and Asian. Study data were obtained via individual chart review of included patients with mortality data supplemented/confirmed by National Death Index search [24]. The study was approved by the Institutional Review Board at Stanford University, Stanford, CA. All authors had access to the study data and reviewed and approved the final manuscript.
The primary study outcomes included the incidence of liver events and overall and non-liver-related mortality. Liver-related outcomes included the development of NAFLD-i (defined as NAFLD with stage 1 fibrosis or higher), cirrhosis, hepatocellular carcinoma (HCC), and/or liver-related deaths, whichever came first. Cirrhosis was defined by liver histology; clinical diagnosis of portal hypertension, platelet <120,000/┬ĄL, history of ascites and/or hepatic encephalopathy; by radiographic findings such as nodular liver contour; or by noninvasive methods (Fibrosure┬«, FIB-4 >3.25, shear wave ultrasound, Fibroscan┬«, or magnetic resonance elastography).
The study observation period began at the time NAFLD was confirmed, and the censor criteria included the development of study outcomes, loss to follow-up, death, or end of the study period, whichever came first.
We described and compared continuous variables among the 4 study groups using the analysis of variance test if the variables followed a normal distribution and the KruskalŌĆōWallis test if not. We reported results for continuous variables as mean (┬▒standard deviation) or median and interquartile range. For categorical variables, we reported data as numbers and percentages (%) and used the Žć2 test to compare values among groups.
We used the KaplanŌĆōMeier methods to determine the incidence of liver-related outcomes, overall mortality, and non-liver-related mortality. We used the log-rank test to compare the incidence of events of interest among the study groups.
We used univariable Cox proportional hazards regression to estimate the unadjusted hazard ratio (HR) and identify potential factors (with P<0.10) to include in the multivariable model to estimate adjusted hazard ratios (aHR) for factors associated with the development of liver events, overall or non-liver related mortality. Factors with potential association with outcomes by prior reports were also included in the multivariable models. Statistical significance was defined with a two-tailed P-value <0.05, and all analyses were done using the Stata version 17 (Stata Corporation, College Station, TX, USA).
Our study cohort included a total of 9,340 NAFLD patients who met our study criteria. The study patients were divided into four groups: White (4,115 patients, 44.1%), Black (214 patients, 2.3%), Hispanic (2,604 patients, 27.9%), and Asian (2,407 patients, 25.7%) (Table 1). Hispanic patients were the youngest group with a mean age of 44.5 years, about 10 years younger than the White patients (mean age 54.1 years), followed by Asian and Black patients (mean age 48.3 and 51.4 years, respectively) (P<0.0001). The Hispanic group was most likely to be female (63.3%) while the majority of patients in the Asian group were males (55.5%) (P<0.0001). Black patients had the highest body mass index (BMI) and the highest percentage of diabetes mellitus, hypertension, cardiovascular disease, and chronic kidney disease. In fact, the majority of Black patients in the cohort had hypertension (65.9%), close to one-half had diabetes mellitus (42.1%), and about one in three (30.4%) had chronic kidney disease. Patients in the Black group had the lowest aspartate aminotransferase, alanine aminotransferase, and the highest platelet levels compared to other groups, but they had the highest alkaline phosphatase (Table 2). However, we found no significant difference in the total cholesterol, triglycerides, or glucose levels among the four racial and ethnic groups. White patients were most likely to have non-liver cancer (23.0% compared to 13.1ŌĆō14.8% in other groups, P<0.0001). The percentage of cirrhosis was lowest in the Asian group (15.5%), followed by the Hispanic group (17.8%), and highest in the White (21.8%) and Black (23.3%) groups (P<0.0001) (Table 1). Notably, while Black patients made up 2.29% of the total cohort, only 0.67% of the liver biopsies were performed in Black patients as compared to 49.11% among White patients who made up 44.06% of the cohort.
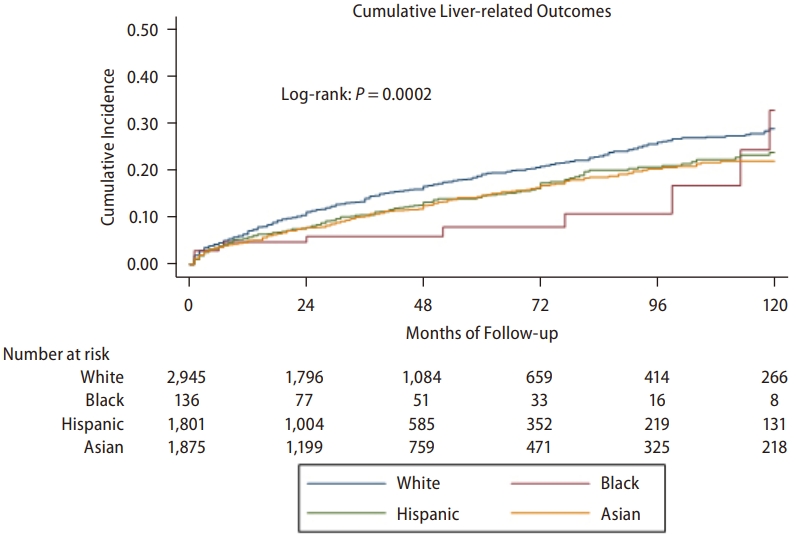
Over a follow-up of 140,167 persons-years for White patients, 5,985 persons-years for Black patients, 76,684 persons-years for Hispanic, and 97,556 persons-years for Asian patients, there were 2,711 liver-related events among White patients, 149 among Black patients, 1,898 among Hispanic patients, and 1,793 among Asian patients. Figure 1 shows that the rate of development of liver-related events differs significantly among the racial and ethnic groups (P=0.0002). The highest cumulative 5-year incidence was observed among White patients (19.1%), the lowest among Black patients (7.9%), and the Asian and Hispanic patients having fairly similar rates (14.6% and 14.5%, respectively). The difference among the White, Hispanic, and Asian groups remained significant even after Black patients, as the group with the lowest rate was excluded (P<0.0001).
On univariable Cox proportional hazard regression, compared to the White group, Black, Hispanic, and Asian groups were all associated with lower risk of NAFLD-i (aHR 0.60 to 0.82), but the association between Black patients and NAFLD-i was not statistically significant (P=0.07) (Table 3). On the multivariable model adjusted for age, sex, race and ethnicity, and diabetes mellitus, compared to White patients, only Asian patients were significantly associated with about 20% lower risk of NAFLD-i (aHR 0.81, 95% confidence interval [CI] 0.70ŌĆō0.95, P=0.008) and cirrhosis (aHR 0.81, 95% CI 0.68ŌĆō0.96, P=0.02). In addition, Hispanic patients had nearly four times greater risk of having liver-related mortality compared to White patients (aHR 3.84, 95% CI 1.63ŌĆō9.04, P=0.002) after adjusting for age, sex, and diabetes mellitus. However, in another multivariable model adjusting for additional comorbidities such as cardiovascular diseases, chronic kidney disease, high BMI, and hyperlipidemia, we found that Black patients were less likely to have NAFLD-i and cirrhosis compared to White patients in this study (Table 3).
Among all death events in this study, two most common causes were cardiovascular-related (32.47%) and non-liver cancer-related (36.16%). In contrast to the findings of liver-related outcomes above, we found much higher overall and non-liver-related mortality rates among Black patients as compared to the other three groups (Fig. 2, P=0.0017 and 0.0004, respectively). Over a follow-up of in persons-years of 205,137 for White, 8,054 for Black, 103,652 for Hispanic, and 122,929 for Asian patients, there were 137, 10, 65, and 49 deaths of any cause, respectively. The 5-year and 10-year cumulative overall mortality was highest for Black patients (9.2% and 15.0%, respectively), about 3 times higher than those of the other groups (3.5% and 7.3% for White, 2.6% and 5.6% for Hispanic, and 2.5% and 4.3% for Asian groups). In a sensitivity analysis excluding Black patients as the group with the highest rate, there remained significant differences in both the overall (P=0.031) and non-liver related (P=0.026) mortality among the White, Hispanic, and Asian groups.
We found that the majority of deaths in all racial and ethnic groups to be non-liver related and similar patterns of differences were among the study groups with non-liver related mortality. The numbers of non-liver-related deaths were 127, 10, 52, and 45 for White, Black, Hispanic, and Asian groups, respectively (corresponding follow-up time in persons-years: 205,137; 8,054; 103,653; and 122,929, respectively). The 5-year and 10-year cumulative rates for non-liver mortality were highest at 9.2% and 15.0% for Black, followed by White (3.2% and 6.5%), and lowest for Hispanic (2.0% and 4.0%) and Asian (2.3% and 3.9%) patients.
On multivariable regression analysis (Table 4), compared to White patients, Black patients were at more than two times higher risk of both non-liver related (aHR 2.35, 95% CI 1.22ŌĆō4.51, P=0.010) as well as overall mortality (aHR 2.13, 95% CI 1.11ŌĆō4.08, P=0.022). Hispanic patients also had about 50% higher risk of overall mortality compared to White patients (aHR 1.44, 95% CI 1.05ŌĆō1.99, P=0.022), but there was no statistically significant difference between Hispanic and White patients in regard to non-liver-related mortality risk (aHR 1.23, 95% CI 0.87ŌĆō1.75, P=0.22). Meanwhile, though not statistically significant, there was a trend for also lower risk of overall (aHR 0.70, 95% CI 0.50ŌĆō1.00, P=0.053) and non-liver related (aHR 0.70, 95% CI 0.48ŌĆō1.01, P=0.059) deaths among Asian patients as compared to White patients. In a sensitivity analysis adjusting for other comorbidities such as BMI, diabetes mellitus, cardiovascular diseases, chronic kidney disease, and hyperlipidemia, we found similar trends and direction to the results described above, except the hazard ratio for overall mortality for Black patients no longer reached statistical significance (Table 4).
Using clinically based individual longitudinal data, we were able to closely examine the association of race and ethnicity and long-term outcomes among patients with NAFLD in this study. We found that although Black patients made up the smallest group of our overall cohort, they carried a significantly higher comorbidity burden compared to White, Hispanic, and Asian patients. As such, this may explain why they also were at the highest risk for overall and non-liver-related mortality despite having a lower incidence of liver-related outcomes. Black patients with NAFLD incurred a two times higher risk for overall and non-liver-related mortality compared to White patients. Hispanic patients followed a similar pattern as Black patients but were at a lower rate compared to Black patients. Asian patients were at a 19% less risk for liver-related outcomes compared to White patients.
Because Black patients were the smallest group but carried such a high comorbidity burden and had the highest prevalence of cirrhosis, we removed them from our sensitivity analysis to determine the impact of race and ethnicity among White, Hispanic, and Asian groups. We found that White patients retained the highest risk for the cumulative incidence of liver-related outcomes, while Hispanic and Asian patients remained similar. White patients also had the highest cumulative incidence of overall and non-liver-related mortality, followed by Hispanic and then Asian patients.
Our data provides further evidence on the prevalence of NAFLD by race and ethnicity, where Black patients tend to comprise a smaller proportion among those with NAFLD [25]. On the other hand, these data provide additional information on long-term outcomes among persons with NAFLD in the United States, an area that has been under-reported due to the use of cross-sectional data. We found that Black patients with NAFLD carry a substantial risk for overall mortality and non-liver-related mortality outcomes, followed by White, Hispanic, and Asian patients. These findings held true after adjusting for the clinical differences between the groups. These results hold significance for policymakers as although Black individuals may have a lower susceptibility to developing NAFLD with fibrosis, but once present, they are disproportionately affected [2,26]. Therefore, continued actions are needed to prevent the development and progression of NAFLD in Black patients and address barriers to healthcare. Hispanic patients also appear to be affected by various social determinants of health that increase their risk of developing NAFLD, so efforts in determining culturally sensitive and appropriate healthy living interventions are needed in these communities [27-31].
These recommendations take on more significance for Hispanic and Black females as they not only comprised the largest group among Black and Hispanic individuals but results from a recent study found that Hispanic and Black females experienced significant increases in the liver transplant wait-list due to non-alcoholic steatohepatitis (NASH) [32]. In fact, this study reported that NASH was the second leading indication for liver transplantation overall but the number one indication among women, especially in Hispanic and Black females. In addition, a previous study highlighted that Black patients who developed HCC after 2010 had worse survival compared to White patients due to their more advanced stage at presentation, while race and ethnicity was not an independent predictor for mortality, highlighting again the need to improve access to healthcare for Black patients [33]. Most importantly, Black patients were significantly more likely to have comorbidities such as higher BMI, hypertension, diabetes mellitus, hyperlipidemia, and cardiovascular and chronic kidney diseases, which are all well-documented risk factors for worse health outcomes and mortality in this group [34-36]. In fact, 60% of the deaths among Black patients in our study were due to cardiovascular diseases. The causes of these disparities are multifactorial and likely due to social and structural determinants of health, such as structural racism and income inequality, that together limit access to care and early diagnosis, education, and intervention [37,38].
On the other hand, Asian patients were at lower risk for NAFLD-i and cirrhosis compared to White patients and marginally at lower risk for overall and non-liver-related mortality compared to White patients. Such findings are in line with what has been reported in prior studies [19,39,40]. In one specific study conducted among patients with HCC, investigators determined that Asian patients had improved survival compared to White patients. The investigators suggested that their improved survival could be due to genetic differences that altered the detrimental effects of factors associated with severe disease development. Although further research is needed to understand this premise among patients with NAFLD, this reasoning may be plausible as Asian patients in our study also had the lowest prevalence of cirrhosis.
Though our study was conducted retrospectively at a single tertiary care center, the cohort was large and racially and ethnically diverse, with a large proportion of Hispanic and Asian patients, and spanned over 25 years. Patients were followed longitudinally, and the study data reflected a collective experience of more than 400,000 person-years. We minimized the risk of selection bias by selecting the cohort consecutively and included patients from all clinics and diverse clinical settings in our healthcare system and not just gastroenterology or liver clinics. With the recent announcement of the metabolic dysfunction-associated steatotic liver disease (MASLD) nomenclature, which highlights the cardiometabolic factors affecting steatotic liver disease, our studies found consistent results with Black patients who had more metabolic risks at presentations had the highest mortality risk compared to patients in other racial and ethnic groups. This further highlights the multifactorial disease pathophysiology of fatty liver disease and metabolic factors as major contributors to worse outcomes. Even though we used the NAFLD definition in our study, these results are still likely applicable with the new MASLD classification, as a recent study has shown that the discrepancy between NAFLD and MASLD is minimal, and findings from NAFLD studies should still be valid even with the nomenclature change [41].
In this large cohort of patients with NAFLD who were followed longitudinally in a major medical center in Northern California, we were able to determine long-term outcomes, including mortality by race and ethnicity. Although Black patients comprised the smallest proportion of our study cohort, they had the worst mortality outcomes. Black patients were at more than 2 times higher risk for both overall and non-liver-related mortality compared to White patients. Hispanic patients were 1.5 times increased risk for overall mortality compared to White patients, while Asian patients were 19% less likely to develop NAFLD-i and cirrhosis. As our understanding of NAFLD pathophysiology is expanded, our findings extend previous reports that used cross-sectional data and provide further evidence that policymakers need to develop interventions that are culturally appropriate and sensitive to the needs of different communities to help improve success.
FOOTNOTES
AuthorsŌĆÖ contribution
Study design: Vy H. Nguyen, Mindie H. Nguyen. Data analysis: Isaac Le, Vy H. Nguyen, Scott Barnet, Mindie H. Nguyen. Data collection: All authors. Drafting of manuscript: Vy H. Nguyen, Isaac Le, Mindie H. Nguyen. Data interpretation, review and revision of manuscript: all authors.
Conflicts of Interest
Mindie H. Nguyen: Research funding: Pfizer, Enanta, Gilead, CurveBio, Exact Sciences, Helio Health, Glycotest, National Cancer Institute, B.K. Kee Foundation, Vir Biotech; Consulting: Gilead, Intercept, GSK, Exact Science, Novartis, Janssen, Bayer.
Ramsey Cheung: Research funding: Gilead, Siemens Healthineers.
Figure┬Ā1.
Cumulative incidence of liver-related events(NAFLD-i, cirrhosis, liver cancer, and/or liver-related death).
Figure┬Ā2.
Cumulative incidence of (A) overall mortality and (B) non-liver related mortality among patients with NAFLD by race and ethnicity. NAFLD, non-alcoholic fatty liver disease.

Table┬Ā1.
Baseline characteristics of patients with non-alcoholic fatty liver disease by race and ethnicity
Table┬Ā2.
Baseline laboratory characteristics of patients with non-alcoholic fatty liver disease by race and ethnicity
Table┬Ā3.
Predictors of NAFLD-i, cirrhosis, hepatocellular carcinoma, and liver-related mortality by race and ethnicity
| Predictors | Number of events | Univariable HR (95% CI) | P-value | Multivariable HR Model 1a (95% CI) | P-value | Multivariable HR Model 2a (95% CI) | P-value | |
|---|---|---|---|---|---|---|---|---|
| NAFLD-i | ||||||||
| White | 504 | 1 | 1 | 1 | ||||
| Black | 13 | 0.60 (0.34ŌĆō1.04) | 0.07 | 0.58 (0.34ŌĆō1.01) | 0.05 | 0.54 (0.31ŌĆō0.94) | 0.03 | |
| Hispanic | 232 | 0.82 (0.70ŌĆō0.96) | 0.01 | 0.99 (0.84ŌĆō1.16) | 0.89 | 1.02 (0.87ŌĆō1.20) | 0.80 | |
| Asian | 245 | 0.72 (0.62ŌĆō0.84) | <0.0001 | 0.81 (0.70ŌĆō0.95) | 0.008 | 0.84 (0.71ŌĆō0.99) | 0.03 | |
| Cirrhosis | ||||||||
| White | 412 | 1 | 1 | 1 | ||||
| Black | 11 | 0.63 (0.35ŌĆō1.15) | 0.13 | 0.61 (0.34ŌĆō1.11) | 0.11 | 0.56 (0.31ŌĆō1.02) | 0.06 | |
| Hispanic | 202 | 0.89 (0.75ŌĆō1.05) | 0.17 | 1.06 (0.89ŌĆō1.26) | 0.51 | 1.10 (0.92ŌĆō1.32) | 0.29 | |
| Asian | 199 | 0.72 (0.61ŌĆō0.85) | <0.0001 | 0.81 (0.68ŌĆō0.96) | 0.02 | 0.85 (0.71ŌĆō1.03) | 0.09 | |
| Hepatocellular carcinomab | ||||||||
| White | 3 | 1 | 1 | 1 | ||||
| Hispanic | 3 | 1.88 (0.38ŌĆō9.30) | 0.44 | 3.38 (0.65ŌĆō17.55) | 0.15 | 3.74 (0.71ŌĆō19.6) | 0.12 | |
| Asian | 2 | 1.15 (0.19ŌĆō6.89) | 0.88 | 1.48 (0.25ŌĆō8.92) | 0.67 | 1.94 (0.30ŌĆō12.43) | 0.48 | |
| Liver-related mortalityb | ||||||||
| White | 10 | 1 | 1 | 1 | ||||
| Hispanic | 13 | 2.64 (1.16ŌĆō6.03) | 0.02 | 3.84 (1.63ŌĆō9.04) | 0.002 | 3.89 (1.51ŌĆō10.03) | 0.005 | |
| Asian | 4 | 0.65 (0.21ŌĆō2.09) | 0.47 | 0.78 (0.25ŌĆō2.51) | 0.68 | 0.88 (0.25ŌĆō3.07) | 0.84 | |
HR, hazard ratios; CI, confidence interval; NAFLD, non-alcoholic fatty liver disease; NAFLD-i, non-alcoholic fatty liver disease with stage 1 fibrosis or higher.
Table┬Ā4.
Predictors of overall mortality and non-liver related mortality among patients with NAFLD by race and ethnicity
| Predictors | Number of events | Univariable HR (95% CI) | P-value | Multivariable HR Model 1a (95% CI) | P-value | Multivariable HR Model 2a (95% CI) | P-value | |
|---|---|---|---|---|---|---|---|---|
| Overall mortality | ||||||||
| White | 137 | 1 | 1 | 1 | ||||
| Black | 10 | 2.05 (1.07ŌĆō3.91) | 0.029 | 2.13 (1.11ŌĆō4.08) | 0.022 | 1.93 (0.96ŌĆō3.85) | 0.06 | |
| Hispanic | 65 | 0.95 (0.71ŌĆō1.30) | 0.77 | 1.44 (1.05ŌĆō1.99) | 0.022 | 1.53 (1.09ŌĆō2.16) | 0.02 | |
| Asian | 49 | 0.57 (0.40ŌĆō0.80) | 0.002 | 0.70 (0.50ŌĆō1.00) | 0.053 | 0.81 (0.55ŌĆō1.20) | 0.29 | |
| Non-liver-related mortality | ||||||||
| White | 127 | 1 | 1 | 1 | ||||
| Black | 10 | 2.22 (1.16ŌĆō4.25) | 0.015 | 2.35 (1.22ŌĆō4.51) | 0.010 | 2.05 (1.02ŌĆō4.12) | 0.04 | |
| Hispanic | 52 | 0.81 (0.57ŌĆō1.13) | 0.227 | 1.23 (0.87ŌĆō1.75) | 0.22 | 1.33 (0.91ŌĆō1.93) | 0.14 | |
| Asian | 45 | 0.56 (0.39ŌĆō0.81) | 0.002 | 0.70 (0.48ŌĆō1.01) | 0.059 | 0.81 (0.53ŌĆō1.22) | 0.31 | |
Abbreviations
aHR
adjusted hazard ratio
ALT
alanine aminotransferase
ANOVA
analysis of variance
AST
aspartate aminotransferase
BMI
body mass index
CLD
chronic liver disease
HCC
hepatocellular carcinoma
HR
hazard ratio
ICD
international classification diagnostics
IQR
interquartile range
LT
liver transplantation
NAFLD
non-alcoholic fatty liver disease
NASH
non-alcoholic steatohepatitis
NDI
national death index
NIT
non-invasive tests
REFERENCES
2. Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40:1387-1395.


3. Miele L, Zocco MA, Pizzolante F, De Matthaeis N, Ainora ME, Liguori A, et al. Use of imaging techniques for non-invasive assessment in the diagnosis and staging of non-alcoholic fatty liver disease. Metabolism 2020;112:154355.


4. M├│zes FE, Lee JA, Selvaraj EA, Jayaswal ANA, Trauner M, Boursier J, et al. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis. Gut 2022;71:1006-1019.



5. Newsome PN, Sasso M, Deeks JJ, Paredes A, Boursier J, Chan WK, et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol 2020;5:362-373.



6. Bril F, Ortiz-Lopez C, Lomonaco R, Orsak B, Freckleton M, Chintapalli K, Hardies J, et al. Clinical value of liver ultrasound for the diagnosis of nonalcoholic fatty liver disease in overweight and obese patients. Liver Int 2015;35:2139-2146.



8. Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de L├®dinghen V, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol 2017;66:1022-1030.


9. de L├®dinghen V, Hiriart JB, Vergniol J, Merrouche W, Bedossa P, Paradis V. Controlled attenuation parameter (CAP) with the XL Probe of the Fibroscan┬«: A comparative study with the M probe and liver biopsy. Dig Dis Sci 2017;62:2569-2577.



10. Gu J, Liu S, Du S, Zhang Q, Xiao J, Dong Q, et al. Diagnostic value of MRI-PDFF for hepatic steatosis in patients with non-alcoholic fatty liver disease: a meta-analysis. Eur Radiol 2019;29:3564-3573.



11. Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology 2019;156:1264-1281 e4.



12. Long MT, Gandhi S, Loomba R. Advances in non-invasive biomarkers for the diagnosis and monitoring of non-alcoholic fatty liver disease. Metabolism 2020;111S:154259.



13. Davison BA, Harrison SA, Cotter G, Alkhouri N, Sanyal A, Edwards C, et al. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J Hepatol 2020;73:1322-1332.


14. Agbim U, Asrani SK. Non-invasive assessment of liver fibrosis and prognosis: an update on serum and elastography markers. Expert Rev Gastroenterol Hepatol 2019;13:361-374.


15. Petroff D, Blank V, Newsome PN, Voican CS, Thiele M, et al. Assessment of hepatic steatosis by controlled attenuation parameter using the M and XL probes: an individual patient data meta-analysis. Lancet Gastroenterol Hepatol 2021;6:185-198.


16. Kechagias S, Nasr P, Blomdahl J, Ekstedt M. Established and emerging factors affecting the progression of nonalcoholic fatty liver disease. Metabolism 2020;111S:154183.


17. Kim D, Kim W, Adejumo AC, Cholankeril G, Tighe SP, Wong RJ, et al. Race/ethnicity-based temporal changes in prevalence of NAFLD-related advanced fibrosis in the United States, 2005-2016. Hepatol Int 2019;13:205-213.



18. Le MH, Devaki P, Ha NB, Jun DW, Te HS, Cheung RC, et al. Prevalence of non-alcoholic fatty liver disease and risk factors for advanced fibrosis and mortality in the United States. PLoS One 2017;12:e0173499.



19. Le MH, Yeo YH, Cheung R, Wong VW, Nguyen MH. Ethnic influence on nonalcoholic fatty liver disease prevalence and lack of disease awareness in the United States, 2011-2016. J Intern Med 2020;287:711-722.



20. Arshad T, Paik JM, Biswas R, Alqahtani SA, Henry L, Younossi ZM. Nonalcoholic fatty liver disease prevalence trends among adolescents and young adults in the United States, 2007-2016. Hepatol Commun 2021;5:1676-1688.




21. Otgonsuren M, Stepanova M, Gerber L, Younossi ZM. Anthropometric and clinical factors associated with mortality in subjects with nonalcoholic fatty liver disease. Dig Dis Sci 2013;58:1132-1140.



22. Flores YN, Yee HF Jr, Leng M, Escarce JJ, Bastani R, Salmer├│n J, et al. Risk factors for chronic liver disease in Blacks, Mexican Americans, and Whites in the United States: results from NHANES IV, 1999-2004. Am J Gastroenterol 2008;103:2231-2238.



23. Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol 2008;49:608-612.


24. NDI. National Center for Health Statistics, Centers for Disease Control and Prevention, Department of Health and Human Services. CDC web site, <https://www.cdc.gov/nchs/ndi/index.htm>. Accessed 29 Jul 2022.
25. Rich NE, Oji S, Mufti AR, Browning JD, Parikh ND, Odewole M, et al. Racial and ethnic disparities in nonalcoholic fatty liver disease prevalence, severity, and outcomes in the United States: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 2018;16:198-210.e2.



26. Setiawan VW, Stram DO, Porcel J, Lu SC, Le Marchand L, Noureddin M. Prevalence of chronic liver disease and cirrhosis by underlying cause in understudied ethnic groups: The multiethnic cohort. Hepatology 2016;64:1969-1977.


27. Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology 2011;53:1883-1894.


28. Agbim U, Carr RM, Pickett-Blakely O, Dagogo-Jack S. Ethnic disparities in adiposity: Focus on non-alcoholic fatty liver disease, visceral, and generalized obesity. Curr Obes Rep 2019;8:243-254.


29. Yang TC, South SJ. Neighborhood effects on body mass: Temporal and spatial dimensions. Soc Sci Med 2018;217:45-54.

30. Leslie T, Pawloski L, Kallman-Price J, Escheik C, Hossain N, Fang Y, et al. Survey of health status, nutrition and geography of food selection of chronic liver disease patients. Ann Hepatol 2014;13:533-540.


31. Paik JM, Mir S, Alqahtani SA, Younossi Y, Ong JP, Younossi ZM. Dietary risks for liver mortality in NAFLD: Global burden of disease data. Hepatol Commun 2022;6:90-100.




32. Noureddin M, Vipani A, Bresee C, Todo T, Kim IK, Alkhouri N, et al. NASH leading cause of liver transplant in women: Updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol 2018;113:1649-1659.




33. Estevez J, Yang JD, Leong J, Nguyen P, Giama NH, Zhang N, et al. Clinical features associated with survival outcome in African-American patients with hepatocellular carcinoma. Am J Gastroenterol 2019;114:80-88.


34. Post WS, Watson KE, Hansen S, Folsom AR, Szklo M, Shea S, et al. Racial and ethnic differences in all-cause and cardiovascular disease mortality: The MESA study. Circulation 2022;146:229-239.



35. Weight N, Moledina S, Volgman AS, Bagur R, Wijeysundera HC, Sun LY, et al. Socioeconomic disparities in the management and outcomes of acute myocardial infarction. Heart 2023 Aug 9. doi: 10.1136/heartjnl-2023-322601.

36. Peterson K, Anderson J, Boundy E, Ferguson L, McCleery E, Waldrip K. Mortality disparities in racial/ethnic minority groups in the veterans health administration: An evidence review and map. Am J Public Health 2018;108:e1-e11.



37. Kardashian A, Serper M, Terrault N, Nephew LD. Health disparities in chronic liver disease. Hepatology 2023;77:1382-1403.




38. Bailey ZD, Feldman JM, Bassett MT. How structural racism works - Racist policies as a root cause of U.S. racial health inequities. N Engl J Med 2021;384:768-773.


39. Golabi P, Paik J, Hwang JP, Wang S, Lee HM, Younossi ZM. Prevalence and outcomes of non-alcoholic fatty liver disease (NAFLD) among Asian American adults in the United States. Liver Int 2019;39:748-757.


-
METRICS

-
- 1 Web of Science
- 0 Crossref
- 0 Scopus
- 2,255 View
- 96 Download
- ORCID iDs
-
Mindie H. Nguyen

https://orcid.org/0000-0002-6275-4989 - Related articles
-
Global incidence and prevalence of nonalcoholic fatty liver disease2023 February;29(Suppl)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



