| Clin Mol Hepatol > Volume 30(2); 2024 > Article |
|
Non-alcoholic fatty liver disease (NAFLD) is a condition characterized by excessive hepatic fat accumulation leading to steatosis in >5% of hepatocytes, in the absence of secondary causes and excessive alcohol consumption [1]. Recent changes in nomenclature adopt ‘positive diagnostic criteria’ to acknowledge metabolic dysfunction underpinning the central pathogenesis of the disease, and the substitution of ‘fatty’ by ‘steatotic’ [2]. Since these entities have >90% overlap with minimal reclassification due to the new nomenclature [3], the rest of the article will use NAFLD for simplicity of presentation. The term NAFLD encompasses a broad spectrum of clinical conditions ranging from non-alcoholic fatty liver (NAFL), non-alcoholic steatohepatitis (NASH), NASH cirrhosis, and hepatocellular carcinoma (HCC). NAFL refers to pure hepatic steatosis without significant lobular inflammation or hepatocyte ballooning. Compared to NAFL, people with NASH are at risk of accelerated disease progression to significant liver fibrosis, cirrhosis or HCC [4,5]. Apart from liver-related morbidities, people with NAFLD are also at a high risk for adverse cardiovascular outcomes and extra-hepatic malignancies [6,7]. The world observes an alarming increase in the global prevalence of NAFLD, affecting 25–40% with regional variations [8,9]. In addition, patients with NAFLD in the West and the East differ due to a multitude of factors, including genetic background, socio-economic status, healthcare coverage, diet, and physical activity [10]. Therefore, it is important to understand the natural course of NAFLD in the context of changing epidemiology.
In this issue, Le et al. [11] conducted a large-scale systematic review and meta-analysis comprising 79 studies, including 1,377,466 subjects with NAFLD, to delineate the outcomes of NAFLD in terms of the incidence of adverse clinical events. The huge sample size allowed the inclusion of cohorts representative of various regions of the world, as well as subgroups with well-characterized clinical phenotypes. The authors defined clinical outcomes as follows: mortality (all-cause, cardiovascular-related, liver-related, and non-liver cancer-related), liver-related outcomes (fibrosis progression, cirrhosis, liver transplant, HCC, decompensation [ascites, varices, hepatic encephalopathy]), metabolic-related events (metabolic syndrome, hypertension, hyperlipidemia, diabetes mellitus), cardiovascular events (coronary artery disease, congestive heart failure, myocardial infarction, ischemic or hemorrhagic stroke, renal impairment, and depression/anxiety), and non-liver cancer. Multiple subgroup analyses were performed to address the impact of biopsy-proven NASH, diagnostic method, geographical location, and era of study to gain insights in each subpopulation.
Consistent with the existing literature, the pooled incidence rate of mortality was highest for cardiovascular-related deaths, followed by non-liver cancer-related deaths, and liver-related mortality being the second runner-up (4.45, 3.27, and 3.10 per 1,000 person-years, respectively). The most striking finding was the regional variations in the incidence of mortality and the cause of mortality. North America and Europe surpassed Western Pacific and Southeast Asia in all-cause mortality, cardiovascular-related mortality, and non-liver cancer-related mortality. On the other hand, Western Pacific and Southeast Asia had a higher incidence of HCC compared to North America and Europe. Apart from diet and genetic polymorphisms, such differences could also be attributed to competing risks for all-cause mortality, cardiovascular-related, and non-liver cancer-related death in North America and Europe, allowing patients in the Western Pacific/Southeast Asia region to survive long enough to develop HCC. The incidence of liver-related mortality was similar across the involved regions.
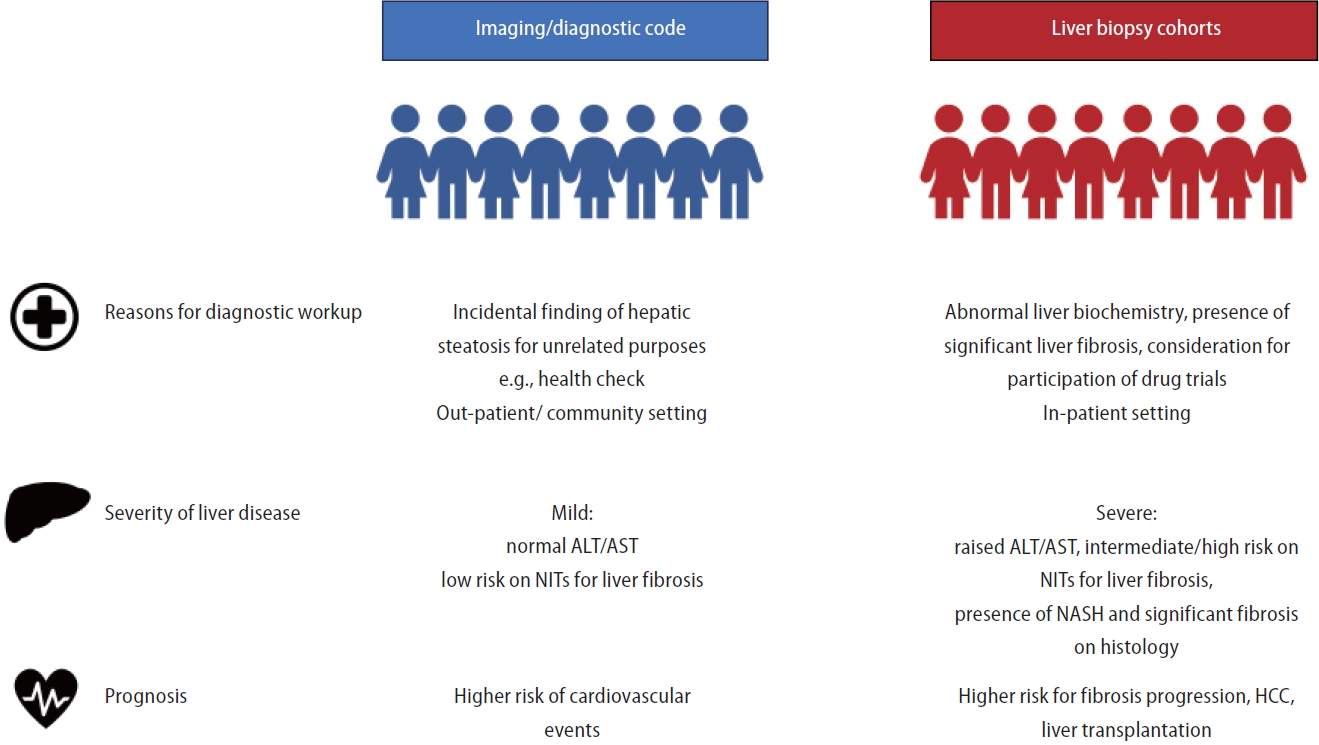
The authors confirmed the implication of biopsy-proven NASH and the presence of cirrhosis, more HCC, liver-related mortality, and liver events with these histological features. The study also confirmed worse clinical outcomes for cohorts that used liver biopsy instead of non-invasive methods such as imaging or diagnostic codes to identify NAFLD–those with clinical indications for liver biopsy, and thus identified as having NAFLD by liver biopsy-demonstrated a higher risk of mortality, fibrosis progression, liver transplant, and HCC. This acknowledges the heterogeneity of clinical outcomes and highlights the fact that NAFLD diagnostic method should be taken into account when interpreting the observed differences in prognosis among NAFLD patients (Fig. 1). Over the past few decades, despite an increasing prevalence of NAFLD, the rates of non-liver events (cardiovascular and non-liver cancer events) have decreased, while those of liver events have increased. Intriguingly, with an increase in study year, the baseline median age decreased (regression coefficient of 0.19 per year). This trend is concerning, whether the underlying mechanism is increased awareness of NAFLD or the obesity pandemic, especially among the younger generation. Patients will start to live with NAFLD at an earlier age, and they also live longer due to improvement in care for cardiovascular health. Overall, the duration of illness (NAFLD) will eventually increase, contributing to ever-growing pool of patients at risk of adverse clinical outcomes.
Intrinsic to the study design, the current meta-analysis could not provide details on how disease modifiers such as genetic polymorphisms and medications influence the disease course of NAFLD. Nevertheless, the study has clearly described the clinical outcomes in a large cohort of NAFLD patients with granular details. Le et al. [11] call for urgent attention to recognize and appreciate the multi-faceted needs of patients living with NAFLD, which is an ever-expanding pool. Clinical care for NAFLD should prioritize to minimizing morbidity and mortality from cardiovascular diseases, cancer, and liver events.
Figure 1.
Accountability of NAFLD diagnostic method in heterogeneous clinical presentation and prognosis among NAFLD patients. NAFLD, non-alcoholic fatty liver disease; ALT, alanine aminotransferase; AST, aspartate aminotransferase; NITs, non-invasive tests; NASH, non-alcoholic steatohepatitis; HCC, hepatocellular carcinoma.
REFERENCES
1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73-84.


2. Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol 2023;79:1542-1556.

3. Song SJ, Lai JC, Wong GL, Wong VW, Yip TC. Can we use old NAFLD data under the new MASLD definition? J Hepatol 2024;80:e54-e56.


4. Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 2015;13:643-654.e1-9 quiz e39-40.



5. Day CP. Natural history of NAFLD: remarkably benign in the absence of cirrhosis. Gastroenterology 2005;129:375-378.


6. Paik JM, Henry L, De Avila L, Younossi E, Racila A, Younossi ZM. Mortality related to nonalcoholic fatty liver disease is increasing in the United States. Hepatol Commun 2019;3:1459-1471.




7. Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015;61:1547-1554.


8. Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2019;4:389-398.


9. Le MH, Le DM, Baez TC, Wu Y, Ito T, Lee EY, et al. Global incidence of non-alcoholic fatty liver disease: A systematic review and meta-analysis of 63 studies and 1,201,807 persons. J Hepatol 2023;79:287-295.


-
METRICS

- ORCID iDs
-
Lung-Yi Mak

https://orcid.org/0000-0002-2266-3935 - Related articles
-
In response to: Steatotic liver disease-know your enemies2024 April;30(2)
Nonalcoholic fatty liver disease and non-liver comorbidities2023 February;29(Suppl)
Epidemiology of alcoholic liver disease in Korea2018 June;24(2)
Socioeconomic costs of liver disease in Korea2011 December;17(4)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



