Changes of guidelines diagnosing hepatocellular carcinoma during the last ten-year period
Article information
Abstract
Hepatocellular carcinoma (HCC) is the third most common cause of cancer deaths in the world. There have been many advances in the diagnosis of HCC during the last ten years, especially in the imaging techniques. The Korean Liver cancer study group (KLCSG), European Association for the Study of the Liver (EASL), American Association for the Study of Liver disease (AASLD), and Asian-Pacific Association for the Study of Liver (APASL) have made and changed the HCC guidelines with the advances in the imaging techniques and according to the results of the researches on HCC. We reviewed the changes of the imaging guidelines in HCC diagnosis according to the advances in the imaging techniques. Further studies will be necessary to resolve the controversies in the diagnosis of HCC smaller than 1 cm in size.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most common cancer in the world and the third most common cause of cancer mortality. Furthermore, Korea is one of the countries with high prevalence.1 HCCs are diagnosed by invasive methods, such as biopsy, and non-invasive methods, including imagings and tumor markers. Since percutaneous biopsy can cause several problems, such as bleeding due to liver dysfunction given that HCC patients often have cirrhosis, difficulties in tumor targeting, and tumor seeding,2 non-invasive methods are preferred in the diagnosis of HCCs. Non-invasive methods include imaging diagnosis such as computed tomography (CT) and magnetic resonance imaging (MRI), and tumor markers, such as alpha-fetoprotein. The great advances made recently in the imaging diagnosis and the results from the studies of these imaging methods have led to changes in the guidelines for HCC diagnosis. We reviewed the changes of the imaging guidelines in HCC diagnosis according to advances in the imaging techniques during the last decade.
European Association for the Study of the Liver (EASL) guideline in 2000 and Korean Liver Cancer Study Group (KLCSG) guideline in 2003
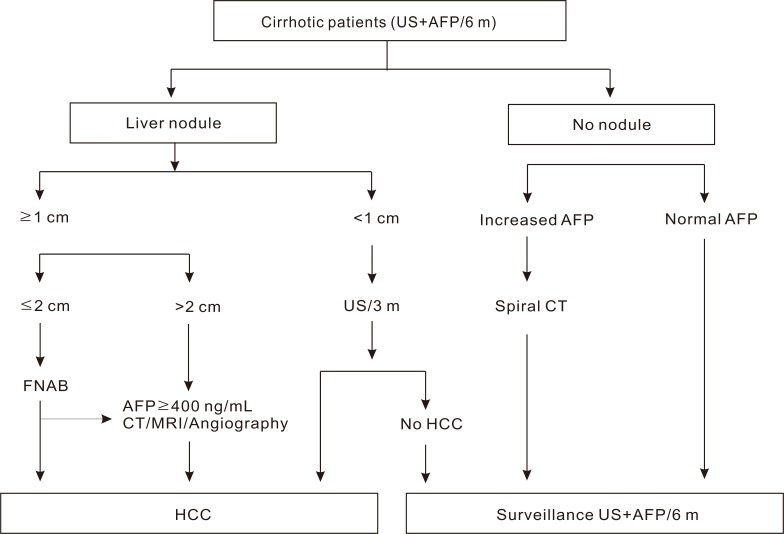
European Association for the Study of the Liver (EASL) guideline on clinical management of HCC in 2000 (Fig. 1).3
-
Radiological criteria: two coincident imaging technique
Focal lesion >2 cm with arterial hypervascularization
-
Combined criteria: one imaging technique associated with AFP
Focal lesion >2 cm with arterial hypervascularization
-
AFP levels >400 ng/mL
Four techniques considered: US, spiral CT, MRI and angiography
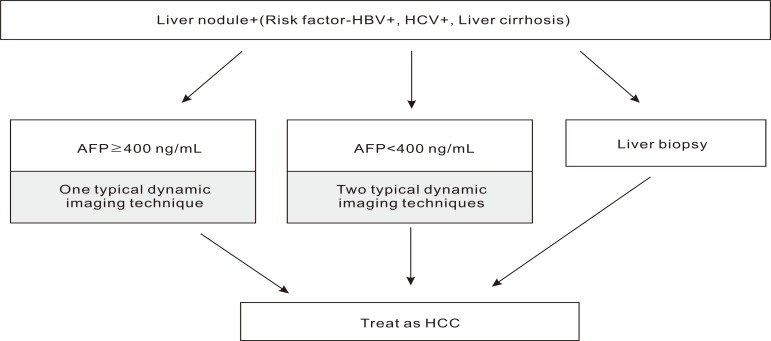
Korean Liver Cancer Study Group (KLCSG) guideline in 2003 (Fig. 2).4
Risk factors such as HBV, HCV, and Liver cirrhosis
AFP levels ≥400 ng/mL: one imaging technique compliant with HCC
-
AFP levels <400 ng/mL: two imaging techniques compliant with HCC
Three techniques are available: Multiphasic spiral CT, Dynamic MRI, and arteriography
According to the algorithms for liver nodule, which were suggested by EASL in 2000, HCCs are diagnosed based on the nodule size, AFP, and radiologic examination. The KLCSG suggested similar guidelines analogous to that of EASL, except that HCCs were diagnosed based on imaging and AFP regardless of tumor size. The imaging techniques specified in KLCSG guideline were multiphasic spiral CT, dynamic MRI, and angiography. KLCSG adopted multiphasic spiral CT and dynamic MRI in its guideline according to their high sensitivities and specificities. The sensitivity and specificity of multiphasic spiral CT were 61-87.7% and 91%. Further, dynamic MRI showed 91-100% sensitivity in tumors larger than 2 cm, while 35-71% sensitivity in tumors less than 2 cm.5-9 In the cohort study10 of HCCs larger than 1 cm and diagnosed by KLCSG guideline, the sensitivity, specificity and positive predictive value were 95.1%, 73.9%, and 93.7%, respectively. In addition, the result that there were no differences in sensitivity, specificity and positive predictive value according to tumor size, supported the KLCSG guidelines algorithm for HCC diagnosis that excluded tumor size as diagnostic criteria.
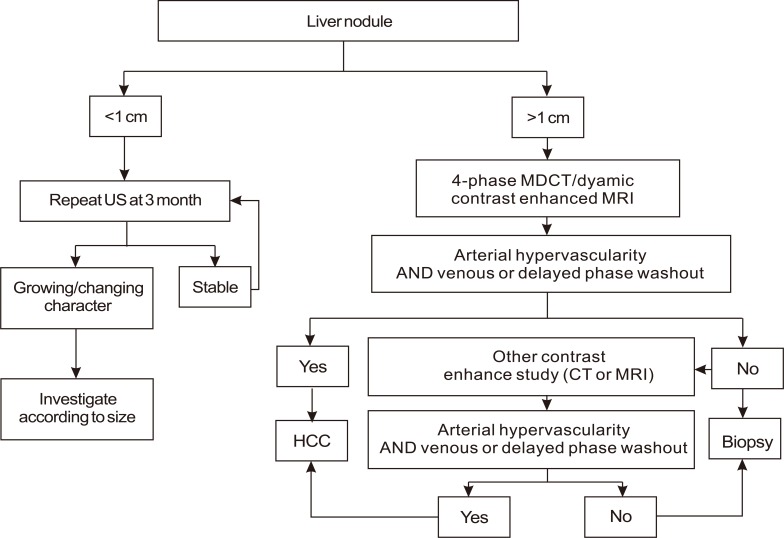
American Association for the Study of Liver Diseases (AASLD) guideline on the management of HCC in 2005 (Fig. 3).11
Nodules found on ultrasound surveillance that are smaller than 1 cm should be followed with ultrasound at intervals from 3-6 months.
Nodules of 1-2 cm found on ultrasound screening of a cirrhotic liver should be investigated further with two dynamic studies, either CT scan, contrast ultrasound or MRI with contrast. If the appearances are typical of HCC (i.e., hypervascular with washout in the portal/venous phase) in two techniques, the lesion should be treated as HCC.
If the nodule is larger than 2 cm at initial diagnosis and has the typical features of HCC on one dynamic imaging technique, biopsy is not necessary for the diagnosis of HCC. Alternatively, if the AFP is >200 ng/mL, biopsy is also not required.
If the vascular profile on imaging is not characteristic or if the nodule is detected in a non-cirrhotic liver, biopsy should be performed.
If the biopsy is negative for HCC, patients should be followed by ultrasound or CT scanning at 3-6 monthly intervals, until the nodule either disappears, enlarges, or displays diagnostic characteristics of HCC.
A guideline that diagnosed HCC according to tumor size, imaging studies, and AFP was suggested by the AASLD in 2005. Compared to the EASL guideline, it was noteworthy that the importance of imaging studies increased while that of biopsy decreased. When the HCCs diagnosed by only imaging studies and AFP without biopsy were compared with those of resected specimens, the positive predictive value was more than 95%.12,13 As venous wash-out in the delayed portal/venous phase was added as typical features of HCC in the AASDL guideline, the diagnostic accuracy increased furthermore. Unlike the EASL guideline in 2000, which had suggested biopsy in the patients with 1-2 cm sized tumors, regardless of their imaging features, the AASLD guideline suggested diagnosing HCCs without biopsy if their appearances were typical in two different imaging techniques. This change in the guidelines represents the increased importance of imaging techniques and the decreased importance of biopsies.
The cut-off level of AFP to diagnose HCC was lowered from 400 ng/mL, which was recommended in the 2000 EASL conference, to 200 ng/mL in the AASLD guidelines. Although AFP is frequently used as screening and diagnostic measures, it is considered to be an inadequate test for screening. Trevisani et al14 reported that the cut-off level of 200 ng/mL with sensitivity 22.4% and specificity 66-97% was superior to the cut-off level of 400 ng/mL with sensitivity 17.1% and specificity 60-96%. Torzilli et al12 reported that sensitivity, specificity and positive predictive value for HCC diagnosis were nearly 99% with the cut-off level of 200 ng/mL. Thus, the cut-off level of AFP for HCC diagnosis was lowered from 400 ng/mL to 200 ng/mL in 2005 AASLD guideline.
New guidelines for hepatocellular carcinoma diagnosis
With the progression in the diagnostic imaging techniques, Korean Liver Cancer Study Group (KLCSG), American Association for the Study of Liver Disease (AASLD), Asia-Pacific Association for the Study of the Liver (APASL), and European Association for the Study of the Liver (EASL), published new guidelines for HCC diagnosis since 2009.
Korean Liver Cancer Study Group (KLCSG) guideline in 2009 (Fig. 4).15
-
When nodules are detected in ultrasound surveillance in a high risk group for HCC (positive for hepatitis B or C virus, or liver cirrhosis), dynamic contrast enhancement CT or MRI should be performed for the diagnosis.
If the serum AFP level is ≥200 ng/mL in high-risk patients, typical characteristic of HCC in either dynamic contrast enhancement CT or dynamic contrast enhancement MRI lead to the diagnosis of HCC.
If the serum AFP level is <200 ng/mL, two or more positive findings of 1) dynamic contrast enhancement CT, 2) dynamic contrast enhancement MRI or 3) hepatic arterial angiography would lead to the diagnosis of HCC.
When a tumor of 2 cm or larger in patients with liver cirrhosis has typical characteristic of HCC in dynamic contrast enhancement CT or MRI, one could diagnose it as HCC regardless of the serum AFP levels.
The lesion does not satisfy the above criteria or shows atypical findings of HCC, biopsy should be performed for the diagnosis.
-
If nodules of high risk patients are smaller than 1 cm, which diagnosis may not be verified by a radiologic or histologic examination, a tumor marker test and ultrasonography should be performed several times, repeatedly, in an interval of three to six months, monitoring for any increase in the size and the level of tumor marker.
Typical characteristics: arterial hypervascularity with wash-out in the portal/venous phase
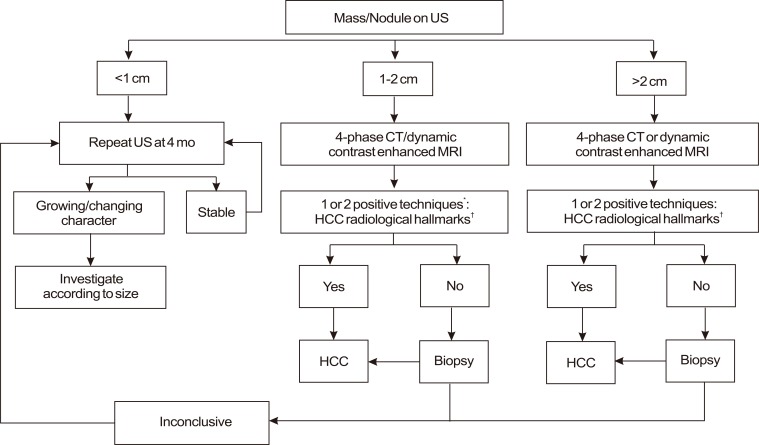
American Association for the Study of Liver Disease (AASLD) guideline in 2010 (Fig. 5).16
-
Nodules larger than 1 cm found on ultrasound screening of a cirrhotic liver should be investigated further with either 4-phase multidetector CT scan or dynamic contrast enhanced MRI.
If the appearances are typical of HCC, the lesion should be treated as HCC.
If the findings are not characteristic or the vascular profile is not typical, a second contrast enhanced study with the other imaging modality should be performed, or the lesion should be biopsied.
Nodules found on ultrasound surveillance that are smaller than 1 cm should be followed with ultrasound at intervals from 3-6 months.
-
If the biopsy is negative for patients with HCC, the lesion should be followed by imaging at 3-6 monthly intervals, until the nodule either disappears, enlarges, or displays diagnostic characteristics of HCC. If the lesion enlarges but remains atypical for HCC a repeat biopsy is recommended.
Typical characteristics: hypervascularity in the arterial phase with washout in the portal venous or delayed phase
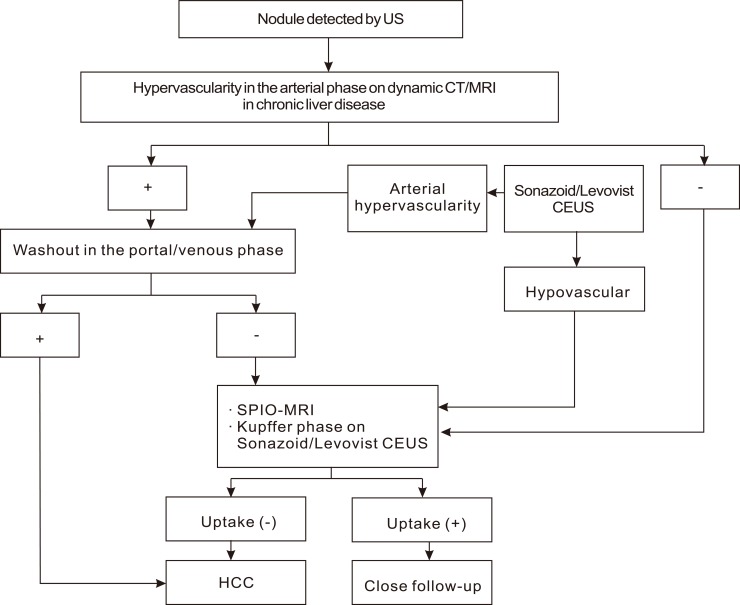
Asia-Pacific Association for the Study of the Liver (APASL) guideline in 2010 (Fig. 6).17
Typical HCC can be diagnosed by imaging regardless of the size if a typical vascular pattern, i.e., arterial enhancement with portal-venous washout, is obtained on dynamic CT, dynamic MRI, or contrast enhanced ultrasonography (CEUS).
Nodular lesions show an atypical imaging pattern, such as iso- or hypo-vascular in the arterial phase or arterial hypervascularity alone without portal-venous washout, should undergo further examinations such as SPIO MRI or CEUS.
European Association for the Study of the Liver (EASL) guideline in 2012 (Fig. 7).18
In cirrhotic patients, nodules less than 1 cm in diameter detected by ultrasound should be followed every 4 months the first year and with regular checking every 6 months thereafter.
In cirrhotic patients, diagnosis of HCC for nodules of 1-2 cm in diameter should be based on non-invasive criteria or biopsy-proven pathological confirmation. A second biopsy is recommended in case of inconclusive findings, or growth or change in enhancement pattern identified during follow-up
In cirrhotic patients, nodules more than 2 cm in diameter can be diagnosed for HCC based on typical features on one imaging technique. In case of uncertainty or atypical radiological findings, diagnosis should be confirmed by biopsy.
-
Non-invasive criteria can only be applied to cirrhotic patients and are based on imaging techniques obtained by 4-phase MDCT scan or dynamic contrast-enhanced MRI. Diagnosis should be based on the identification of the typical hallmark of HCC. While one imaging technique is required for nodules beyond 1 cm in diameter, a more conservative approach with 2 techniques is recommended in suboptimal settings.
Typical hallmark: hypervascular in the arterial phase with washout in the portal venous or delayed phases.
Compared with the 2003 KLCSG guideline, the changes in the 2009 KLCSG guideline were
Cut-off value of AFP was lowered from 400 ng/mL to 200 ng/mL.
Tumor which is 2 cm or larger and with typical characteristics of HCC in dynamic contrast enhancement CT or MRI could be diagnosed as HCC regardless of the serum AFP level in the patients with liver cirrhosis on the basis that a few reports6,7,19 suggested that the diagnostic accuracy of HCC on imaging was 100% with tumors 2 cm or larger.
Also, according to the result20 that the tumor showing a typical contrast pattern on a single imaging modality should be diagnosed as HCC, 2010 AASLD guideline suggested that nodules of 1 cm or larger could be diagnosed with HCC by one typical imaging. AFP was excluded from the diagnostic criteria in the 2010 AASLD guideline because the sensitivity of AFP for HCC diagnosis was lower than that of ultrasonography in tumors less than 3 cm, and the combination of AFP and ultrasonography was not cost-effective due to the increased false positive rate. However, based on the randomized controlled trial that surveillance with AFP coupled with ultrasonography reduced the mortality of HCC,21 the claim that AFP should be included in the HCC surveillance guidelines of AASLD is gaining a lot of support.22 Thus, whether AFP should be included in the guideline will have to be concluded by a large randomized controlled trial.
According to the 2010 APASL guideline, HCC is diagnosed by dynamic imaging technique regardless of the tumor size and AFP. When the arterial enhancement is not definite in the dynamic contrast enhance CT or MRI, contrast enhanced ultrasound (CE-US) can be helpful in diagnosing HCC.23,24 As the HCC nodule has fewer kupffer cells than the non-tumor tissue, HCC can be diagnosed by CEUS and SPIO-MRI, using contrast taken up by the Kupffer cells. As biopsy and tumor marker were excluded from the HCC diagnosis algorithm and a positive result in just one radiologic examination lead to the diagnosis of HCC, the significance of imaging technique has further grown in the 2010 APASL guideline.
The 2012 EASL guideline, published lately, is similar to the 2010 AASLD guideline. AFP is not included as a surveillance nor a diagnostic tool, and HCC can be diagnosed by one imaging technique. However, in suboptimal settings, where the technology at disposal or the local skills are not at the high-end level, this guideline recommends using at least two coincidental techniques because of high rates of false positive diagnosis above 10%.25 On the other hand, the pathologic diagnosis is recommended for the cases with inconclusive or atypical imaging appearance in cirrhotic livers and for all nodules developing in non-cirrhotic livers.
In the recent guidelines for HCC diagnosis, the importance in biopsy and AFP have reduced and that of imaging has increased based on the high accuracy of up to date radiologic modalities. Tumors less than 1 cm is very difficult to diagnose, and therefore, they are screened by ultrasound at the interval of 3-6 months in many cases. However, in Korea, the consensus on the diagnosis of HCC less than 1 cm by using mutidetector row computed tomography (MDCT) and hepatocyte specific contrast MRI were achieved. Therefore, guidelines on the diagnosis of the tumors 1 cm or smaller is expected to be developed in the future.
Imaging techniques for HCC diagnosis
With the advancement in the imaging techniques, various radiologic examinations are used for the diagnosis of HCC.
Contrast enhanced ultrasonography (CEUS) can diagnose HCC by using contrast with microbubble that provide contrast between HCC nodule and hepatic parenchyma. Because of hemodynamic characteristics of HCC, CEUS shows enhancement at the arterial phase and wash-out at portal and delayed phase, similar to that of the contrast enhanced CT and MRI. According to Forner et al,26 Dai et al,27 Trillaud et al,28 and Hatanaka et al,29 the diagnostic performance of CEUS was similar to that of MDCT or dynamic MRI. Furthermore, unlike iodinated contrast agents for CT and gadolinium chelates for MR imaging, US contrast agents are not cleared by the kidneys and are very safe with a low incidence of side-effects. However, since a comprehensive assessment of the whole liver parenchyma cannot be accomplished during the short duration of the arterial phase, CT or MR imaging are still mandatory for proper intrahepatic staging of the disease.30
In multi-detector row CT (MDCT), the number of detector row has been increased from single detector row CT (SDCT) to 64 or 128. Increased detector row allows faster acquisition, thinner slices and repetitive imaging during multiple perfusion phases after contrast material injection. This made it possible to reconstruct three dimensional or multiplane imaging and the higher spatial and temporal resolution of the MDCT has led to the achievement of a higher detection rate.31 The sensitivity of HCC diagnosis was increased from 37-54% of SDCT to 65-79% of MDCT.19
The most commonly used contrast in the dynamic MRI is the extracellular contrast, gadolinium. After intravenous administration, gadolinium distributes in the extracellular space, exhibits no tissue specificity, and is excreted by the kidneys. HCC can be diagnosed by triphasic imaging acquired during the arterial, portal and delayed phase, based on the differences in the hemodynamics of tumor and non-tumor. In addition, the detection rate of HCC is increasing after the invention of the hepatocyte specific contrast agent such as Gadobenate (Gd-BOPTA), gadoxetic acid (Gd-EOB-DTPA) and reticuloendothelial agent that are taken up by Kupffer cells, such as SPIO (superparamagnetic iron oxide). Hepatocyte specific contrasts distribute in the extracellular space and are excreted by kidney like gadolinium in the early phase after administration. However, they help to detect HCC by increasing the contrast between tumor and non-tumor as they are taken up by hepatocyte and excreted by biliary system in the late phase. SPIO is a reticuloendothelial contrast agent that is taken up by the Kupffer cells and its signal intensity is reduced in the T2-weighted image. HCC nodule lacks Kupffer cell activity hence fail to take up SPIO. Therefore, HCC lesion will show high signal intensity compared to normal liver after SPIO administration and this technique will be particularly helpful in diagnosing hypovascular HCCs.
The sensitivity and specificity for HCC diagnosis, through the review of several literatures, were 60% and 93% in US, 68% and 93% in CT, 81% and 85% in MRI, respectively, and MRI showed the highest specificity.32 However, the diagnostic sensitivity of each imaging technique depends on the size of the tumor. The sensitivity of CT and MRI for HCC was more than 90% in tumor of 2 cm or larger, 61-65% and 80-92% in tumor between 1 and 2 cm, 10% and 34-71% in tumor less than 1 cm, respectively. The smaller the tumor size, more difficult it is to diagnose HCC.
CONCLUSION
Many advances have been made in the diagnosis of HCC during the recent ten years or so, with the imaging technique at the core of such advances. With the progression in the imaging techniques, such as ultrasonography, CT, and MRI and the accumulation of experiences of the radiologists, the accuracy in the diagnosis of HCC along with the significance of the diagnostic imaging has increased, whereas the importance of tumor marker and invasive method such as biopsy has declined. In this regard, many changes have been made in the guideline of HCC diagnosis. Though tumor with less than 1 cm-size is still recommended to be kept followed-up regularly without definite diagnosis, a new guideline is apt to be developed in the near future through continuous investigation and discussion.
Notes
The authors have no conflicts to disclose.
Abbreviations
AASLD
American Association for the Study of Liver Disease
AFP
alpha-fetoprotein
APASL
Asia-Pacific Association for the Study of the Liver
CEUS
contrast enhanced ultrasonography
CT
computed tomography
EASL
European Association for the Study of Liver
HCC
hepatocellular carcinoma
KLCSG
Korean Liver Cancer Study Group
MDCT
multi-detector row computed tomography
MRI
magnetic resonance imaging
SPIO
superparamagnetic iron oxide