| Clin Mol Hepatol > Volume 19(2); 2013 > Article |
ABSTRACT
Background/Aims
Spontaneous bacterial peritonitis (SBP) has been known to greatly influence the survival rate of patients with liver cirrhosis. However, the factors that affect the survival rate in patients with SBP need to be clarified.
Methods
This study enrolled 95 liver cirrhosis patients diagnosed with SBP. The laboratory findings of their serum and ascitic fluid were examined and the characteristics of the isolated microorganisms in their peritoneal fluid were analyzed.
Results
The proportion of patients with culture-positive SBP was 41.1%, and 47 microorganisms were isolated from the ascitic fluid. The proportions of cultured bacteria that were Gram negative and Gram positive were 57.4% and 40.4%, respectively. The proportions of Escherichia coli, Klebsiella species, and Streptococcus species were 25.5%, 19.1%, and 19.1%, respectively. Enterococcus species represented 12.8% of the microorganisms cultured. The overall survival rates at 6, 12, and 24 months were 44.5%, 37.4%, and 32.2%, respectively. There was no relationship between the bacterial factors and the survival rate in SBP. Multivariate analysis revealed that the presence of hepatocellular carcinoma (HCC; P=0.001), higher serum bilirubin levels (≥3 mg/dL, P=0.002), a prolonged serum prothrombin time (i.e., international normalized ratio >2.3, P<0.001), renal dysfunction (creatinine >1.3 mg/dL, P<0.001), and lower glucose levels in the ascitic fluid (<50 mg/dL, P<0.001) were independent predictive factors of overall survival rate.
Liver disease, including cirrhosis, is one of the leading causes of morbidity and mortality in Korea and ranked the 8th most common cause of death in 2007.1 Spontaneous bacterial peritonitis (SBP) is a bacterial infection of the ascitic fluid and is diagnosed based on the following criteria: the presence of more than 250 neutrophils in ascitic fluid, which is not associated with surgery or an intraabdominal origin of infection in liver cirrhosis patients.2,3 SBP is the most common type of infection in hospitalized cirrhotic patients, occurring in about 9% of cases and accounting for about 25% of all infections.4 About 10% of cirrhotic patients with ascites may develop SBP within a year.5 Despite the use of sensitive methods, only 39-41% of culture-positive SBP are in Korean patients with SBP compared to around 60% in Western studies.1,6,7 Among positive culture results, both Gram positive and negative organisms can be isolated, the more common being Gram negative. Common Gram negative organisms are E. coli , Klebsiella pneumoniae and Aeromonas , while Streptococci and Enterococci comprise most of the Gram positive organisms.6,7 When SBP was first described in the 1960s, its prognosis was extremely poor, with in-hospital mortality rate reaching 100%.8 Currently, the outcome of SBP has considerably improved due to the introduction of effective antibiotics and the appropriate use of prophylactic antibiotics to high risk patients of SBP.6,7 Nevertheless, the recent growing percentage of antibiotic-resistant strains remains a serious medical problem in many countries.1 Particularly, the proportion of Gram negative organisms that are resistant to quinolones and that produce the extended spectrum ß-lactamase (ESBL) have increased significantly.1,6 In one study, ESBL-producers are associated with higher mortality and treatment failure rate compared to those not producing ESBL.2 In addition, the proportion of Staphylococci, another factor known to be associated with higher mortality rate, is increasing according to recent studies.9 Despite these facts, the studies that focused on the association between the cultured bacteria species and the clinical outcome of the SBP patients are still limited. The aim of this study was to investigate whether the cultured bacteria species are associated with the poor outcome in liver cirrhosis patients with SBP. In addition, we tried to evaluate other predictive factors for mortality in cirrhotic patients with SBP.
From January 1st 2003 through December 1st 2010, the study included patients with both liver cirrhosis and SBP at Seoul Paik Hospital. Diagnosis of cirrhosis was established using clinical and ultrasonographic findings. SBP was diagnosed based on a polymorphonuclear leukocyte (PMN) count ≥250 cells/mm3 in ascitic fluid in the absence of any clinical and radiologic findings suggestive of secondary peritonitis.10 We excluded patients who showed a sign of free air in their abdominal X-ray and had a recent history of surgery or trauma which increased the possibility of secondary peritonitis rather than SBP. We also excluded patients with severe cardiopulmonary disease or cardiovascular disease, evidence of severe immunosuppression, and other combined malignancies except hepatocellular carcinoma (HCC).
All of the patients' data were collected retrospectively from their electronic and paper medical records. This study was approved by the Institutional Review Board of Seoul Paik Hospital.
Ascitic fluid culture using diagnostic paracentesis was performed in all patients with ascites who developed local symptoms or signs of peritoneal infection, systemic signs of infection, such as fever or leukocytosis, or clinical deterioration without any obvious precipitating factors. Ascitic fluid and peripheral blood were placed in blood culture bottles, incubated at 37℃ for 7 days, and examined daily for turbidity. At least 10 mL of ascitic fluid was inoculated into 2 bottles for aerobic and anaerobic cultures. All isolated organisms in the culture were tested for antimicrobial susceptibility according to the diffusion methods.
The empirical antibiotic was administered immediately when SBP was diagnosed. Follow up ascitic fluid tapping was performed if the signs and symptoms of SBP failed to disappear after 48 hours of initial empirical antibiotics therapy. The resolution of SBP was defined as a fading of all signs and symptoms of SBP or PMN count in ascitic fluid had reduced to <250 cells/mm3. Treatment failure was defined as persistent or worsening of the signs and symptoms of SBP or less than 25% decreased of PMN count in the ascitic fluid tapped 48 hrs after the treatment when compared with that from the first tapped ascites. The initial empirical antibiotic used was a third-generation cephalosporin (cefotaxime). When initial treatment had failed, antibiotic therapy was then changed based on the susceptibility of the cultured organisms to the antibiotics. When initial antibiotic treatments had failed when the ascitic fluid culture came out negative, we switched antibiotics from the 3rd generation cephalosporin (cefotaxime) to a combination of vancomycin and carbapenem (meropenem) considering the presence of ESBL-producing organisms and methicillin-resistant Staphylococcus aureus (MRSA).
Cumulative survival rates were calculated using Kaplan-Meier analysis and the difference was determined by the log-rank test. To find the predictors for survival, multivariate analysis using a proportional hazards Cox regression model was performed. Cause of liver cirrhosis, Child-Pugh grade, MELD (model for end-stage liver disease) score, serum laboratory findings including serum prothrombin time (INR), bilirubin, and albumin levels, cultured bacteria (isolated microorganisms, Gram stain of cultured bacteria, numbers of cultured bacteria), laboratory findings of ascitic fluid, and presence of recurrence of SBP were used on multivariate analysis. MELD score was determined using an online calculator (available at http://www.mayoclinic.org/meld/mayomodel6.html) with the three components of score including serum bilirubin, creatinine, and prothrombin time (INR). A value of P<0.05 was considered statistically significant. All analyses were performed using SPSS version 16.0 (SPSS Inc, Chicago, IL, USA).
A total of 95 liver cirrhosis patients diagnosed with SBP were recruited. 82 patients were diagnosed with SBP for the first time and 13 had a previous history of SBP. Among 13 patients, 4 patients had not received prophylactic antibiotic therapy and 9 patients did not have medical records about the antibiotic therapy because they had been transferred to our hospital after the diagnosis of SBP.
The patients consisted of 70 men (73.7%) and 25 women (26.3%) with a mean age of 58.5±11.8 years. The most common cause of liver cirrhosis was hepatitis B, which was found in 46 patients (48.4%), followed by alcohol abuse in 27 patients (28.4%), hepatitis C in 10 patients (10.5%) and cryptogenic in 12 patients (12.6%). According to the Child-Pugh classification, 2 patients (2.1%) were classified as grade A, 29 (30.5%) were grade B, 64 (67.4%) were grade C. Among 92 patients in whom the MELD scoring was available, the patients with a score higher than or equal to 20 and lower than 20 were found in 51 (55.4%) and 41 (44.6%), respectively. The presence of HCC at the time of diagnosis of SBP was noted in 39 patients (41.5%). Recurrence of SBP was found in 10 of the 95 patients (Table 1).
Serum and ascitic fluid were sampled at the diagnosis of SBP. The levels of albumin (g/dL), bilirubin (mg/dL), prothrombin time (INR), and creatinine (mg/dL) in serum and the levels of PMN count (cells/mm3), protein (g/dL), lactate dehydrogenase (LDH) (IU/L), and glucose (mg/dL) in ascitic fluid were obtained. These laboratory findings are summarized in Table 1.
Microorganisms in ascitic fluid were isolated in 39 patients (41.1%) and a total of 47 species of microorganisms were isolated. Among the 39 patients, 9 patients (23.1%) had 2 or more species of microorganisms. Gram negative organisms were found in 20 patients (51.3%), Gram positive organisms were found in 13 patients (33.3%) and 6 patients (15.4%) had both Gram positive and negative organisms in their ascitic fluid. Among the 47 isolated microorganisms, 27 (57.4%) were Gram negative organisms, 19 (40.4%) were Gram positive organisms, and 1 (2.2%) was a Candida species. E. coli was the most frequently isolated organism (12 of 47 cases, 25.5%), followed by Klebsiella species (9 cases, 19.1%), Streptococcus species (9 cases, 19.1%), and Enterococcus species (6 cases, 12.8%). Table 2 shows a profile of the isolated microorganisms. The patient who had spontaneous fungal peritonitis with candida in ascitic fluid (categolized in SBP according to the definition of SBP) was in terminal stage with metastatic HCC and died due to hepatic failure shortly after diagnosis of SBP.
Median follow up period for survival after diagnosis of SBP was 2.5 months (range, 0.1-60 months). The in-hospital mortality rate was 38.9%. The overall cumulative survival rates at 6 months, 12 months and 24 months were 44.5%, 37.4%, and 32.2%, respectively. In univariate analysis, the presence or absence of the bacteria, the types of isolated microorganism, the Gram stain of the bacteria, and the number of isolated microorganisms all did not significantly affect the survival rate of patients with SBP. Survival rate was also not associated with serum albumin levels. Moreover, no statistical significance was found between the laboratory findings of the peritoneal fluid and the survival rates of the patients. On the other hand, patients with Child-Pugh score ≥10 showed significantly lower survival rate than patients with Child-Pugh score <10 (P=0.019; Fig. 1). In addition, patients with MELD score higher than or equal to 20 had significantly lower survival rates than patients with <20 (P=0.001; Fig. 2). The patients with higher serum bilirubin (≥3 mg/dL) showed higher mortality than patients with <3 mg/dL (P=0.012; Fig. 3). Prolonged serum prothrombin time (INR) also had a significant influence on the survival rate when the interval was categorized as <1.7, 1.7-2.3 and >2.3 (P<0.001; Fig. 4). Renal dysfunction (serum creatinine >1.3 mg/dL; normal range, 0.7-1.3 mg/dL; P<0.001; Fig. 5), the presence of HCC at the time of diagnosis of SBP, and lower glucose levels in ascitic fluid (<50 mg/dL) influenced the survival rates. The survival rates according to the variable factors in univariate analysis are summarized in Table 3. In multivariate analysis, the presence of HCC at the time of diagnosis of SBP (OR, 3.305; P=0.001), higher serum bilirubin levels (≥3 mg/dL; OR,3.872; P=0.002), the prolonged serum prothrombin time (INR) (>2.3; OR, 4.117; P=0.001), renal dysfunction (serum creatinine >1.3 mg/dL; OR, 3.752; P<0.001), and lower glucose levels in ascitic fluid (<50 mg/dL; OR, 28.849; P<0.001) were the independent predictive factors of overall survival rates in cirrhotic patients with SBP (Table 4).
In the present study, the microorganisms were isolated in 39 of 95 patients (41.1%). The result was similar to the previous studies conducted in Korea (39-41%) which is lower than that of the Western studies (~60%).11 Only 12-18% of organisms were Gram positive in the 1990s and the proportion increased to 24.1% in 2007 in Korea.7 Similarly, in Western studies, the proportion was 19-34%.12-14 However, in our study, 19 out of 47 isolated microorganisms (40.4%) were Gram positive organisms which was much higher than that in previous studies. Although Eschericia coli (12 of the 47 cases, 25.5%), Klebsiella species (9 cases, 19.1%) and Streptococcus species (9 cases, 19.1%) were still the most common organisms in this study, Enterococcus species (6 cases, 12.8%) was noticeably higher in our study compared to the previous studies in Korea and Western countries.1,15-18 One possible explanation that might account for the higher proportion of Gram positive organisms in the present study, compared to the previous studies, is the wide abuse of prophylactic antibiotics in cirrhotic patients with SBP; however, we could not conclude that prophylactic antibiotic therapy is associated this phenomenon because prophylactic antibiotic therapy was not performed or not known in their medical records of 13 patients with a previous history of SBP.
It is widely accepted that the prophylaxis of SBP should be performed in patients with an acute gastrointestinal hemorrhage, a low total protein content in ascitic fluid or a previous history of SBP due to high recurrence rates.6,19 One study showed that patients with serum bilirubin >2.5 mg/dL also had a higher incidence of SBP recurrence rates, which explains for the need for prophylaxis of SBP.20 Despite the documented evidences of high recurrence rates, the studies that focus on the factors that influence the mortality of liver cirrhosis patients with SBP are limited. This is why the aim of this study was to investigate the factors that influence the survival rates of patients with SBP.
In the literature, SBP caused by ESBL-producing organisms or Staphylococcus species was identified as factors closely associated with high mortality.9,21,22 However, despite the relatively low proportion of ESBL-producing organisms (n=1) and Staphylococcus species (n=3), our data showed that the types of cultured bacterial organisms did not affect the survival rates of cirrhotic patients with SBP. Our data also compared the relationship between the presence and absence of the bacteria, the positivity of bacterial Gram stain, the number of isolated microorganisms, and the mortality rates. However, no statistical significances were found. Moreover, the laboratory findings of the ascitic fluid including PMN, protein, and LDH did not show any association with the survival of the patients.
A multicenter retrospective study performed in Korea at a comparatively recent date reported that high mortality rates were seen in the patients with a high MELD score. In addition to MELD score, ESBL producing organisms-induced SBP and combined HCC were associated with poor prognosis in SBP patients.21 The other study reported that bacteremia, higher MELD score, and no microbiological response were prognostic factors for a poor outcome in SBP patients.23 Recently, Tandon and Garcia-Tsao reviewed 18 prognostic studies for in-hospital and 1 month mortalities in adult patients with SBP. In their review, renal dysfunction was the most important independent predictor for mortality in cirrhotic patients with SBP, followed by the MELD score.24
In this study, there was no relationship between the bacterial factors at the time of diagnosis of SBP and the survival rate; on the other hand, patients with Child-Pugh score ≥10 showed significantly lower survival rates than patients with Child-Pugh score <10. MELD score higher than or equal to 20 was also strongly associated with high mortality. However, both higher Child-Pugh and MELD score were not significant independent predictors of survival in SBP patients. In multivariate analysis, the presence of HCC at the time of diagnosis of SBP, higher serum bilirubin levels, the prolonged serum prothrombin time (INR), renal dysfunction at the diagnosis of SBP, and lower glucose levels in ascitic fluid were the independent predictive factors of overall survival rates in cirrhotic patients with SBP.
There were several limitations in this study. First, it was a single center study. Therefore, it unlikely reflects all of the characteristics of isolated organisms in Korea. Second, the number of patients involved in this study was relatively small. Third, the follow-up periods after diagnosis of SBP were short; we even failed to obtain follow-up records of 10 patients for sufficiently long duration. Fourth, we could not have the assessment of treatment response in many patients, which was not available in multivariate analysis, owing to the retrospective characteristic of this study. Fifth, the in-hospital mortality and cumulative mortalities at 6, 12, and 24 months in this study was high compared to the previous results, which may be influenced by the fact that the percentage of patients with combined HCC was high up to 41.5%.
In conclusion, the proportion of Gram positive organisms, especially Enterococcus species, is increasing. The bacterial factors including the presence or absence of the bacteria, the types of isolated microorganism, the positivity of bacterial Gram stain and the number of isolated microorganisms did not influence the survival rate of patients with SBP. The presence of HCC and laboratory findings at the time of diagnosis of SBP including higher serum bilirubin level, the prolonged serum prothrombin time (INR), renal dysfunction at the diagnosis of SBP, and lower glucose level in ascitic fluid were the independent predictive factors of mortality in cirrhotic patients with SBP.
Acknowledgements
This work was supported by Grant from Inje University, 2005. This research was also supported by the GlaxoSmithKline Research Fund of the Korean Association for the Study of the Liver (KASL) in 2007 (Soo Hyung Ryu, MD).
REFERENCES
1. Jang JW. Current status of liver diseases in Korea: liver cirrhosis. Korean J Hepatol 2009;15(Suppl 6):S40-S49. 20037279.


2. Lee JM, Han KH, Ahn SH. Ascites and spontaneous bacterial peritonitis: an Asian perspective. J Gastroenterol Hepatol 2009;24:1494-1503. 19743995.


3. Saab S, Hernandez JC, Chi AC, Tong MJ. Oral antibiotic prophylaxis reduces spontaneous bacterial peritonitis occurrence and improves short-term survival in cirrhosis: a meta-analysis. Am J Gastroenterol 2009;104:993-1002. 19277033.


4. Garcia-Tsao G. Ascites. In: Boyer TD, Wright TL, Manns MP, eds. Zakim and Boyer's hepatology, A Textbook of Liver Disease. Vol 1: 5th Ed. Philadelphia: Saunders & Elsevier; 2006. p. 333-346.
5. Wang SS, Tsai YT, Lee SD, Chen HT, Lu CW, Lee FY, et al. Spontaneous bacterial peritonitis in patients with hepatitis B-related cirrhosis and hepatocellular carcinoma. Gastroenterology 1991;101:1656-1662. 1659549.


6. European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol 2010;53:397-417. 20633946.


7. Park MK, Lee JH, Byun YH, Lee Hle, Gwak GY, Choi MS, et al. Changes in the profiles of causative agents and antibiotic resistance rate for spontaneous bacterial peritonitis: an analysis of cultured microorganisms in recent 12 years. Korean J Hepatol 2007;13:370-377. 17898553.


8. Kerr DN, Pearson DT, Read AE. Infection of ascitic fluid in patients with hepatic cirrhosis. Gut 1963;4:394-398. 14084751.



9. Campillo B, Richardet JP, Kheo T, Dupeyron C. Nosocomial spontaneous bacterial peritonitis and bacteremia in cirrhotic patients: impact of isolate type on prognosis and characteristics of infection. Clin Infect Dis 2002;35:1-10. 12060868.



10. Guarner C, Soriano G. Spontaneous bacterial peritonitis. Semin Liver Dis 1997;17:203-217. 9308125.



11. Song HG, Lee HC, Joo YH, Jung S, Park YH, Ryu SH, et al. Clinical and microbiological characteristics of spontaneous bacterial peritonitis (SBP) in a recent five year period. Korean J Hepatol 2002;8:61-70.
12. Boixeda D, De Luis DA, Aller R, De Argila CM. Spontaneous bacterial peritonitis. Clinical and microbiological study of 233 episodes. J Clin Gastroenterol 1996;23:275-279. 8957729.


13. Dupeyron C, Campillo B, Mangeney N, Richardet JP, Leluan G. Changes in nature and antibiotic resistance of bacteria causing peritonitis in cirrhotic patients over a 20 year period. J Clin Pathol 1998;51:614-616. 9828822.



14. Laroche M, Harding G. Primary and secondary peritonitis: an update. Eur J Clin Microbiol Infect Dis 1998;17:542-550. 9796651.


15. Koulaouzidis A, Bhat S, Saeed AA. Spontaneous bacterial peritonitis. World J Gastroenterol 2009;15:1042-1049. 19266595.



16. Koulaouzidis A, Bhat S, Karagiannidis A, Tan WC, Linaker BD. Spontaneous bacterial peritonitis. Postgrad Med J 2007;83:379-383. 17551068.



17. Garcia-Tsao G. Spontaneous bacterial peritonitis. Gastroenterol Clin North Am 1992;21:257-275. 1568776.


18. Runyon BA. Ascites and spontaneous bacterial peritonitis. In: Feldman M, Friedman LS, Sleisenger MH, eds. Sleisenger and Fordran's gastrointestinal and liver disease. 8th Ed. Philadelphia: Saunders; 2006. p. 1935-1964.
19. Runyon BA. Low-protein-concentration ascitic fluid is predisposed to spontaneous bacterial peritonitis. Gastroenterology 1986;91:1343-1346. 3770358.


20. Novella M, Sola R, Soriano G, Andreu M, Gana J, Ortiz J, et al. Continuous versus inpatient prophylaxis of the first episode of spontaneous bacterial peritonitis with norfloxacin. Hepatology 1997;25:532-536. 9049193.


21. Heo J, Seo YS, Yim HJ, Hahn T, Park SH, Ahn SH, et al. Clinical features and prognosis of spontaneous bacterial peritonitis in Korean patients with liver cirrhosis: a multicenter retrospective study. Gut Liver 2009;3:197-204. 20431746.



22. Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Oh MD, et al. Clinical outcome of bacteremic spontaneous bacterial peritonitis due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Korean J Intern Med 2004;19:160-164. 15481607.



Figure 1
Cumulative survival rates according to the Child-Pugh score in SBP patients. A Child-Pugh score of ≥10 reflects a poor survival.

Figure 2
Cumulative survival rates according to the Model for End-Stage Liver Disease (MELD) score in SBP patients. A MELD score of ≥20 reflects a poor survival.
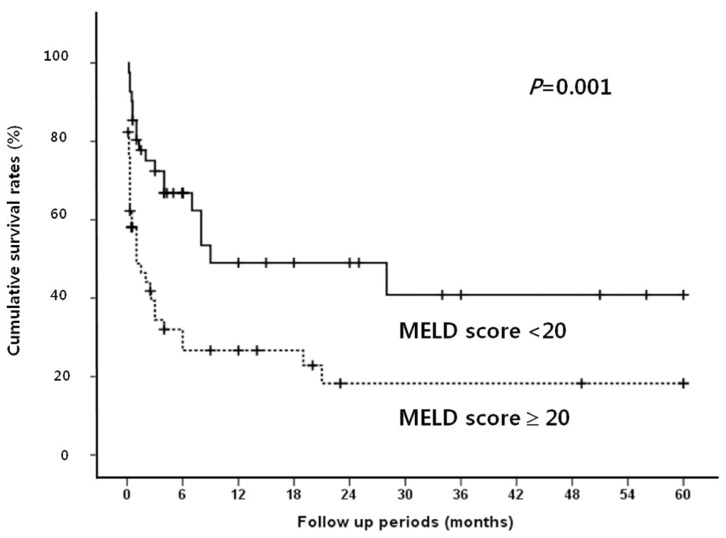
Figure 3
Cumulative survival rates according to serum bilirubin level in SBP patients. A serum bilirubin level of ≥3 mg/dL is related to a poor prognosis.

Figure 4
Cumulative survival rates according to serum prothrombin time [international normalized ratio (INR)] in SBP patients. A prolonged serum prothrombin time (INR of >2.3) is related to poor prognosis.
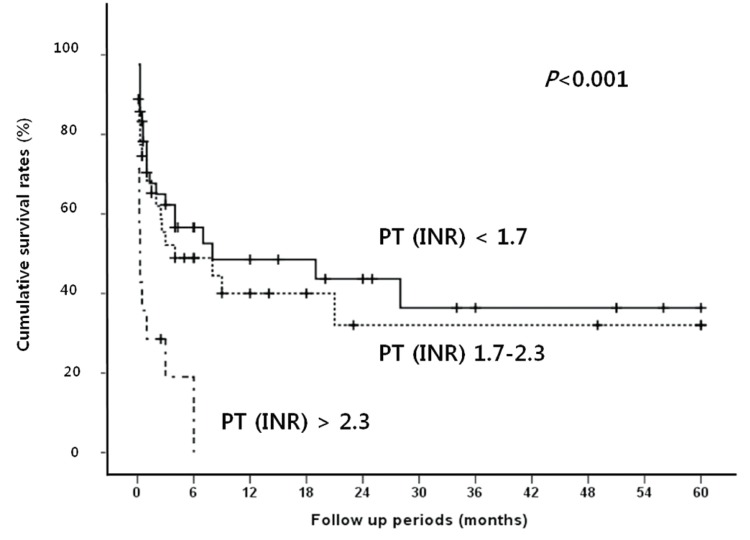
Figure 5
Cumulative survival rates according to serum creatinine level in SBP patients. Renal dysfunction is associated with poor survival.
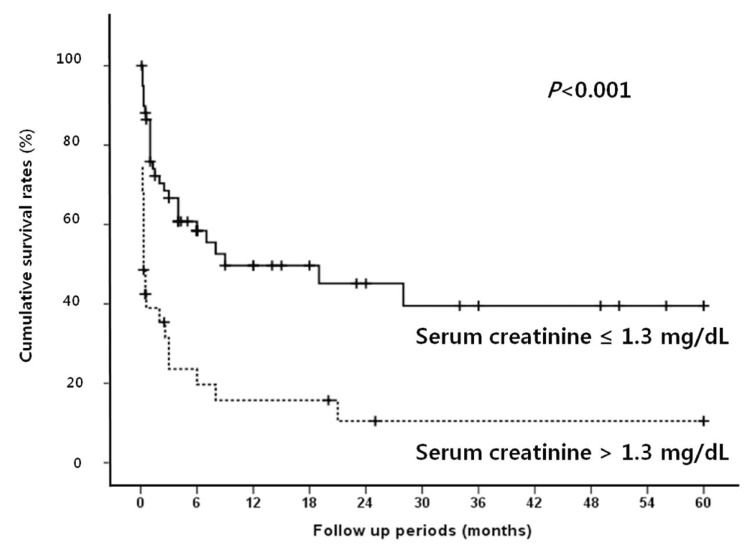
Table 1.
Baseline characteristics of the enrolled patients
| Patients | (n=95) |
|---|---|
| Gender (M/F) | 70/25 |
| Age (yr, mean±SD) | 58.5±11.8 |
| Cause | |
| HBV | 46 (48.4%) |
| HCV | 10 (10.5%) |
| Alcohol | 27 (28.4%) |
| Cryptogenic | 12 (12.6%) |
| Child-Pugh class | |
| A | 2 (2.1%) |
| B | 29 (30.5%) |
| C | 64 (67.4%) |
| MELD score* | |
| <20 | 41 (44.6%) |
| ≥20 | 51 (55.4%) |
| Combined HCC† | |
| Yes | 39 (41.5%) |
| No | 55 (58.5%) |
| Laboratory findings‡ | |
| Serum | |
| Albumin (g/dL) | 2.5 (1.6-3.7) |
| Bilirubin (mg/dL) | 3.9 (0.3-38.1) |
| Prothrombin time (INR) | 1.7 (1.0-4.6) |
| Creatinine (mg/dL) | 1.1 (0.5-8.1) |
| Ascitic fluid | |
| PMN (cells/mm3) | 1,290 (250-25,592) |
| Protein (mg/dL) | 893 (228-3,780) |
| LDH (IU/L) | 79 (19-9,400) |
| Glucose (mg/dL) | 157 (0-570) |
Table 2.
Profiles of the microorganisms isolated from ascitic fluid
Table 3.
Results of univariate analysis for survival in cirrhotic patients with SBP
| n (%) | P-value | |
|---|---|---|
| Gender | 0.688 | |
| Male | 70 (73.7%) | |
| Female | 25 (26.3%) | |
| Age | 0.903 | |
| ≥60 | 39 (41.1%) | |
| <60 | 56 (58.9%) | |
| Cause | 0.020 | |
| HBV | 46 (48.4%) | |
| HCV | 10 (10.5%) | |
| Alcoholic | 27 (28.4%) | |
| Cryptogenic | 12 (12.6%) | |
| Child-Pugh class | 0.026 | |
| A | 2 (2.1%) | |
| B | 29 (30.5%) | |
| C | 64 (67.4%) | |
| MELD score* | 0.002 | |
| <20 | 41(44.6%) | |
| ≥20 | 51(55.4%) | |
| Combined HCC* | 0.031 | |
| Yes | 39 (41.5%) | |
| No | 55 (58.5%) | |
| 1st diagnosis of SBP | 0.139 | |
| Yes | 82 (86.3%) | |
| No | 13 (13.7%) | |
| Recurrence of SBP | 0.578 | |
| Yes | 10 (10.5) | |
| No | 85 (89.5) | |
| Serum albumin (g/dL) | 0.217 | |
| ≥2.8 | 29 (30.5%) | |
| <2.8 | 66 (69.5%) | |
| Serum bilirubin (mg/dL) | 0.017 | |
| <3.0 | 37 (38.9%) | |
| ≥3.0 | 58 (61.1%) | |
| Serum prothrombin time (INR)* | 0.001 | |
| <1.7 | 42 (45.7%) | |
| 1.7-2.3 | 36 (39.1%) | |
| >2.3 | 14 (15.2%) | |
| Serum creatinine (mg/dL) | <0.001 | |
| ≤1.3 | 60 (63.2%) | |
| >1.3 | 35 (36.8%) | |
| Ascitic PMN (cells/mm3) | 0.456 | |
| <1,000 | 41 (43.2%) | |
| ≥1,000 | 54 (56.8%) | |
| Ascitic protein (mg/dL)* | 0.343 | |
| <1,000 mg | 47 (56.6%) | |
| ≥1,000 mg | 36 (43.4%) | |
| Ascitic LDH (IU/L) * | 0.587 | |
| <225 | 64 (81.0%) | |
| ≥225 | 15 (19.0%)) | |
| Ascitic glucose (mg/dL)* | 0.044 | |
| <50 | 2 (2.5%) | |
| ≥50 | 77 (97.5%) | |
| Presence/absence of bacteria | 0.218 | |
| Presence | 39 (41.1%) | |
| Absence | 56 (58.9%) | |
| Gram stain | 0.657 | |
| Positive | 13 (33.3%) | |
| Negative | 20 (51.3%) | |
| Both | 6 (15.4%) | |
| Types of isolated microorganisms | 0.651 | |
| E. coli | 12 (26.7%) | |
| Klebsiella | 9 (20.0%) | |
| Streptococcus | 9 (17.8%) | |
| Enterococcus | 6 (13.3%) | |
| Others | 11 (22.2%) | |
| Numbers of cultured bacteria | 0.914 | |
| ≥2 | 9 (23.1%) | |
| 1 | 30 (76.9%) |
Table 4.
Results of multivariate analysis for survival in cirrhotic patients with SBP



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




