| Clin Mol Hepatol > Volume 21(3); 2015 > Article |
ABSTRACT
Background/Aims
Hepatitis-B-related acute-on-chronic liver failure has a poor prognosis. However, the advent of potent oral antiviral agents means that some patients can now recover with medical treatment. We aimed to identify the prognostic factors for hepatitis-B-related acute-on-chronic liver failure including the initial as well as the dynamically changing clinical parameters during admission.
Methods
Sixty-seven patients were retrospectively enrolled from 2003 to 2012 at Samsung Medical Center. The patients were classified into three categories: Recovery group (n=23), Liver transplantation group (n=28), and Death group (n=16). The Liver transplantation and Death groups were combined into an Unfavorable prognosis group. We analyzed the prognostic factors including the Model for End-Stage Liver Disease (MELD) scores determined at 3-day intervals.
Results
A multivariable analysis showed that the unfavorable prognostic factors were a high initial MELD score (≥28) (odds ratio [OR] =6.64, p=0.015), moderate-to-severe ascites at admission (OR=6.71, P=0.012), and the aggravation of hepatic encephalopathy during hospitalization (≥grade III) (OR=15.41, P=0.013). Compared with the baseline level, significant reductions in the MELD scores were observed on the 7th day after admission in the Recovery group (P=0.016).
Chronic hepatitis B (CHB) virus infection is one of the most common causes of liver disease, affecting more than 400 million people worldwide. Nucleos(t)ide analog (NA) therapy has dramatically improved the outcomes of CHB in the last 15 years.1 However, it is estimated that 600,000 deaths per year are still reported in patients with CHB.2 Liver-related mortality in CHB can be classified into three categories: (i) complications of liver cirrhosis or hepatocellular carcinoma (HCC), (ii) gradual hepatic decompensation with liver failure, and (iii) acute decompensation, otherwise known as acute-on-chronic liver failure (ACLF).3
ACLF is defined as an acute hepatic insult manifesting as jaundice and coagulopathy, complicated within 4 weeks by ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease. The clinical and immunological features of ACLF are differentiated from those of acute liver failure.4 HBV flares occur in 40-50% of hepatitis B envelope antigen (HBeAg)-positive CHB patients and in 15-30% of HBeAg-negative CHB patients. Up to 8% of these patients develop hepatitis B related acute-on-chronic liver failure (ACLF-HBV).5 The prognosis of ACLF-HBV has been reported to be very poor, with 3-month mortality rates of over 50% without liver transplantation.3 Although liver transplantation has been recognized as the only definitive therapy, some patients can survive with medical treatment alone due to development of potent oral antiviral agents.
Identification of prognostic factors for ACLF-HBV patients is critical, because emergent liver transplantation (LT) is not readily available in many cases because of organ shortage. Previously proposed factors associated with adverse outcomes of ACLF-HBV include pre-existing cirrhosis, prolonged prothrombin time (PT), elevated bilirubin, low albumin level, low platelet count and age.67 Most of these are baseline characteristics estimated at initial admission. Because of the rapid progressive course of ACLF, both the initial static features and the early dynamic changes in clinical features after admission can be helpful to predict the outcome. However, there is a shortage of studies evaluating the effectiveness of changing clinical events after hospitalization in combination with baseline characteristics.
Therefore, the aims of this study were to identify the prognostic factors including both the baseline upon admission and the clinical features during hospitalization in ACLF-HBV patients.
We retrospectively enrolled a total of 141 patients with ACLF in CHB who had been hospitalized at Samsung Medical Center, Seoul, Korea between January 2003 and December 2012. Of these patients, we excluded 74 with the following conditions: 6 patients superinfected with other hepatotropic viruses (hepatitis A [n=5] and hepatitis C [n=1]), 13 patients with combined etiologies of acute liver injury (alcohol (n=4), hepatotoxic drugs including medicinal herbs (n=3), sepsis-related liver injury (n=5), and cryptogenic causes (n=1)), 55 patients with coexistent malignancy (hepatocellular carcinoma (n=54) and esophageal cancer (n=1)). Consequently, 67 patients were analyzed in this study (Fig. 1).
ACLF was defined based on the recommendations from the Asian Pacific Association for the Study of the Liver (APASL): acute hepatic insult manifesting as jaundice (total bilirubin >5 mg/dl) and coagulopathy (prothrombin time international normalized ratio (INR) >1.5), complicated within 4 weeks by ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease.4 CHB diagnosis was based on a history of positive hepatitis B virus surface antigen (HBsAg) for at least 6 months. Acute exacerbation of CHB was confirmed if accompanied by hepatitis B viral load ≥2,000 IU/mL as determined by real-time polymerase chain reaction (PCR) using the COBAS TaqMan HBV quantitative test (Roche Molecular Systems Inc. Branchburg, NJ, USA). Since HBV DNA level was tested using hybridization and sandwich enzyme immunoassay (Digene Hybrid Capture II System, USA) before 2007 in our institution, we unified the unit of viral DNA load to IU/mL with a conversion equation (HBV DNA 20,000 IU/mL=105 copies/ml=0.35 pg/mL).8 The present study was approved by the Institutional Review Board of Samsung Medical Center. Because this study was designed as a retrospective study, all included patients' information was anonymized and deidentified prior to analysis instead of informed consent.
According to the outcome, all patients were classified into 3 categories: (1) the 'Recovery group' had recovered from acute hepatic decompensation with supportive management only, (2) the 'LT group' underwent LT for ACLF after admission, and (3) the 'Death group' had died due to fulminant hepatic failure without receiving LT. We defined recovery from acute hepatic decompensation if a patient survived more than 26 weeks after the occurrence of acute decompensation without LT.9 We separated the above three groups into two: a 'Favorable prognosis group (FG)' versus an 'Unfavorable prognosis group (UFG)'. The Recovery group belonged to the FG while the LT and Death groups were classified as UFG.
We evaluated the clinical and biochemical parameters speculated to reflect the outcomes of ACLF in CHB. Baseline characteristics included age, gender, platelet count, prothrombin time, aspartate aminotransferase (AST), alanine aminotransferase (ALT), serum albumin, creatinine, the presence of HBeAg, HBV DNA level, the presence of liver cirrhosis, hepatic encephalopathy, ascites, hepatorenal syndrome, Child-Pugh classification, and model for end-stage liver disease (MELD) score.10 Liver cirrhosis was defined as coarse appearance of the hepatic parenchyma with surface nodularity on ultrasonography or computer tomography (CT). Also, cirrhosis was assumed in case of varices or splenomegaly with coarse hepatic parenchyma even if liver surface nodularity was not prominent. Grading of hepatic encephalopathy was based on the West Haven criteria.11 Ascites was diagnosed using abdominal ultrasonography or abdomen CT as the presence of fluid in the peritoneal cavity. The term ascites, in all definitions, refers to grade 2 or 3 clinically-detectable ascites (grade 1: mild ascites; grade 2: moderate ascites; and grade 3: massive or tense ascites). Hepatorenal syndrome (HRS) was defined when renal insufficiency developed in advanced liver disease without an apparent cause of renal insufficiency.12
Besides changes in the biochemical parameters above, we evaluated the aggravation of hepatic encephalopathy, development of the systemic inflammatory response syndrome (SIRS) or HRS. SIRS was diagnosed by the presence of 2 or more of the following: (I) temperature over 38℃ or below 36℃; (II) heart rate greater than 90 beats per minute; (III) tachypnea greater than 20 breaths per minute or PaCO2 less than 4.3 kPa; (IV) white cell count greater than 12×109/L or less than 4×109/L or the presence of more than 10% immature neutrophils.13 Also, the change in MELD score during hospitalization was assessed.
Categorical variables are described as numbers and percentages, and continuous values are presented as median values with the ranges. Comparisons between two groups were performed using the Chi-square test or Fisher's exact test for categorical variables, then analyzed using Student's t-test for continuous variables. If continuous variables did not satisfy a normal distribution, the Mann-Whitney test was used. Univariable and multivariable logistic regression were performed to identify the risk factors for unfavorable groups of ACLF in CHB. The choice of variables for multivariable analysis was based on the results of univariable analysis and clinical correlation. A forward conditional stepwise method was used to avoid the multi-collinearity in multivariable analysis. A linear mixed model was used to estimate the differences in MELD score changes during hospitalization for each prognosis group (FG versus UFG). The changes in MELD score were determined from values obtained at 3-day intervals for 2 weeks after hospitalization. Prognosis group and time after hospitalization were fixed effects, and patients were random effects. All analyses were performed using The Statistical Package for Social Sciences (SPSS 20.0, SPSS Inc., Chicago, IL, USA) and a value of P<0.05 was considered statistically significant.
Among the final 67 patients, 49 (73%) were males, and the median age was 48 (19-71). All 67 enrolled patients were separated into 3 categories: the Recovery group (n=23), the LT group (n=28), and the Death group (n=16). These three groups were re-classified into two groups according to the patients' prognosis: a Favorable prognosis group (FG) (n=23) and an Unfavorable prognosis group (LT group plus Death group) (UFG) (n=44).
There were 6 immunosuppressed individuals in the study population. Three patients in the LT group had a treatment history of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy for diffuse large B-cell lymphoma (DLBL). In the Death group, 1 patient had rheumatoid arthritis and was taking an immunosuppressing agent, 1 patient had idiopathic thrombocytopenic purpura and was taking a steroid, and 1 patient had a treatment history of R-CHOP chemotherapy for DLBL.
The median duration of hospitalization was 45 (range 6-194) days in the recovery group. In the LT group, the median duration between admission and liver transplantation was 18 (range 2-80) days. LT was performed using deceased donor liver transplantation for 7 patients, and living donor liver transplantation for the others. The median duration from admission to death was 25 (range 5-66) days in the Death group.
Oral nucleos(t)ide analogue (NA) antiviral agents were used in a total of 61 patients. In the Recovery group, all patients received NAs; 7 (30%) with entecavir (ETV), 15 (65%) with lamivudine (LAM) and 1 with clevudine. In the LT group, 25 (89%) patients received NAs; 11 (39%) with ETV, 10 (36%) with LAM, 2 with adefovir (ADV), 1 with a combination of LAM/ADV and 1 with a combination of LAM/ETV/ADV. In the Death group, 13 (80%) patients received NAs; 5 (30%) with ETV and 8 (50%) with LAM.
We compared clinical and biochemical parameters between FG and UFG. FG patients were significantly younger than UFG patients (45 vs. 49 years, P=0.034). There were also significant differences between the two groups in the initial presence of moderate to severe ascites (P=0.002) and hepatorenal syndrome (P=0.024), serum total bilirubin level (P<0.001), prothrombin time (P<0.001), the rate of Child-Pugh class B or C (P=0.024) and initial MELD score (P<0.001). Gender, the presence of liver cirrhosis, initial severe hepatic encephalopathy (above grade III) and the level of alanine/aspartate aminotransferase, albumin, and creatine were similar between the two groups.
Next, a comparison between the LT and Death groups was performed. There was no significant difference between the two groups except for the initial serum albumin level, which was lower in the Death group (P=0.048).
The aggravation of hepatic encephalopathy, from mild grade (0-II) to severe grade (III-IV), occurred more frequently in the UFG (P<0.001). SIRS (P=0.122) and infection (P=0.068) developed more frequently in the UFG without statistical significance. Variceal bleeding (P=0.290) and new onset of HRS (P=0.044) developed during hospitalization only in the UFG. Among patients with initial HRS or new HRS, continuous renal replacement therapy (CRRT) was applied to 9 (21%) UFG patients (P=0.023).
In subgroup analysis of UFG, there were significant differences in the occurrence of aggravated hepatic encephalopathy (P=0.03), SIRS (P=0.005) and infection (P=0.005) between the LT and Death groups.
Although the mean initial MELD score of the UFG was higher than that of the FG, initial MELD scores showed diffuse overlapped distribution in both groups. We measured the MELD scores of enrolled patients at 3-day intervals (Fig. 2). The serial mean MELD scores were similar at about 24 in the FG. On the other hand, a slight increase in the serial mean MELD scores was noted in the UFG. However, there were some missing serial MELD scores in both groups. Therefore, we compared the changes in subsequent MELD scores during hospitalization using a linear mixed model. There was a significant interaction effect between time and group in the MELD score changes between the FG and UFG (P=0.001). The mean MELD scores predicted by the linear mixed model are shown in Fig. 3. The predicted scores decreased over time in the FG, but increased continuously in the UFG. We used the paired t-test to estimate the changes in mean MELD scores between day 1 and subsequent days to obtain the delta MELD score. The numbers on the bar in Fig. 3 indicate the delta MELD score, the P-value from the paired t-test and the number of included patients. In FG, there was a significant decrease in the MELD score starting from the 7th day of hospitalization. The decrease in delta MELD score was -1.9 on the 7th day (P=0.016), -2.6 on the 10th day (P=0.005), and -2.7 on the 13th day (P=0.023). In UFG, a significant increase in delta MELD score appeared on the 7th day with an increase of 3 (P=0.010), followed by an increase of 4.8 on the 10th day (P=0.000), and 5.5 on the 13th day (P<0.001).
We analyzed the risk factors for unfavorable prognosis in ACLF-HBV (Table 3). A univariable analysis showed that unfavorable prognosis was associated with high levels of initial total bilirubin (≥25 mg/dL) (Odds ratio [OR] 4.74, P=0.008), high initial MELD score (≥28) (OR 6.96, P=0.001), the initial presence of moderate to severe ascites (OR 6.10, P=0.001), the presence of initial hepatorenal syndrome (OR 9.23, P=0.039), the aggravation of hepatic encephalopathy (≥grade III) (OR 31.78, P=0.001) and the development of infection (OR 3.10, P=0.044). A multivariable analysis demonstrated that the risk factors were initial high MELD score (≥28) (OR 6.64, P=0.015), the initial presence of moderate to severe ascites (OR 6.71, P=0.012) and the aggravation of hepatic encephalopathy (≥grade III) during hospitalization (OR 15.41, P=0.013).
For acute-on-chronic liver failure (ACLF) in CHB patients, earlier diagnosis and identification of poor prognosis predictors are necessary to distinguish the patients who require transplantation from those who will survive with only medical care. The objectives of this study are to identify the risk factors for unfavorable outcomes in hepatitis B related acute-on-chronic liver failure (ACLF-HBV) and to observe and compare the changing clinical manifestations of different outcome groups. Our data demonstrates that both baseline clinical features and changing clinical presentations during hospitalization could suggest unfavorable outcomes and the need for earlier LT consideration.
Although ACLF is believed to be reversible, the reversibility depends on the severity and nature of the acute insulting cause and the extent of the underlying chronic liver disease.4 One of the causes of ACLF in CHB is acute exacerbation of CHB, a disease presentation distinct from other types of chronic liver diseases due to its sudden virologic surge followed by biochemical flares. Its pathophysiology and management are also very different from acute liver failure by other causes.3 More than 50% of ACLF-HBV patients will die without LT. LT is the only definitive therapy for ACLF-HBV patients who do not improve with only medical treatment. Therefore, prognostic models to predict spontaneous recovery from ACLF-HBV should be established to select appropriate candidates for LT and to prevent avoidable LT.
Previous studies proposed some predictive scoring models for ACLF such as Child-Turcotte-Pugh (CTP), Acute Physiology and Chronic Health Evaluation (APACHE), Sequential Organ Failure Assessment (SOFA) and Model of End-stage Liver Disease (MELD) scores. However, these studies did not consider the different etiologies of ACLF.414 Furthermore, some regression models using various factors believed to be related to adverse outcomes were proposed to be superior to both the MELD score and the CTP score.1516 However, these studies estimated only the predictive value of initial clinical features. In addition, unfortunately, none of these models demonstrated consistently reliable accuracy in predicting the outcome of ACLF-HBV. Therefore, we investigated the baseline characteristics of ACLF-HBV patients upon admission as well as the changing clinical presentations during hospitalization.
Previous studies identified several baseline factors that were positively associated with mortality in multivariable analysis: hepatic encephalopathy, age, the MELD score, bilirubin level, prolonged prothrombin time, and HBV DNA level.7 The effect of nucleos(t)ide analog therapy such as ETV and LAM is still controversial.171819 Our results are almost comparable to those of the above studies. The significantly different baseline characteristics between FG and UFG were age, ascites (≥grade 2), hepatorenal syndrome, serum bilirubin level, prothrombin time, pre-existing cirrhosis of Child-Pugh class B or C and MELD score. However, the type of nucleos(t)ide analog therapy was not related to the outcome. Furthermore, initial HBV DNA level did not show significant distinction. These data imply that ACLF-HBV is a distinguishable disease entity from acute hepatitis B or chronic hepatitis B for which the key point of treatment is viral suppression.
In addition to the baseline characteristics of ACLF-HBV, our study suggested that dynamic changing clinical features could be more useful for predicting the prognosis. By multivariable analysis, the aggravation of hepatic encephalopathy to severe grade, not the presence of initial severe hepatic encephalopathy, is an independent poor prognosis predictor for ACLF-HBV. Although we could not find a significant difference in infection rate in FG and UFG, SIRS and infection developed at a significant rate in the Death group compared to the LT group according to subgroup analysis of UFG. The hepatic dysfunction in UFG may play a role in the increased susceptibility to infection.20 In spite of a similar degree of initial hepatic dysfunction in the LT and Death groups, the additional organs affected by infection were important in determining the possible condition for LT. Based on this result, if signs of infection appeared during hospitalization in ACLF-HBV patients, early consideration of LT as well as active control of the infection could be helpful to reduce the 3-month mortality rate.
Recent studies demonstrated extremely high short-term mortality (≥90%) in ACLF patients with MELD scores ≥30 under conventional medications.1421 However, variable cut-off values for the initial MELD score ranging from 25 to 30 have been proposed.7 Our multivariable analysis results demonstrated that an initial MELD score over 28 was a significant risk factor. However, initial MELD scores of FG (17-33) showed wide overlapped distribution with those of UFG (14-53). Therefore, there is insufficient evidence to decide on the necessity for LT using only the initial MELD score. Starting from the 7th day of hospitalization, the MELD scores increased by more than 3 in UFG and decreased by more than 1.9 in FG. These results suggest that serial measurement of MELD scores could be helpful for predicting outcomes and selecting a management plan. Although there were statistical limitations in deducing the absolute scale of the delta MELD score or the timing due to the small study population, we were able to confirm the likelihood of an unfavorable outcome with an increase of more than 3 in the MELD score on the 7th day of hospitalization. A decrease in the MELD score of more than 1.9 on the 7th day of hospitalization was found to be related to a favorable outcome. Lee WC et al.21 measured the MELD scores of 51 non-cirrhotic ACLF-HBV patients and presented estimated weekly changes in the MELD scores. The respective increase and decrease in MELD scores for patients who spontaneously recovered and those who required LT was analogous to our data. However, we measured the serial MELD scores of most cirrhotic ACLF-HBV patients at a shorter interval due to the early dynamicity of ACLF and compared the analytic predicted value of the serial MELD scores.
Our study had some limitations. First, this was designed as a retrospective study. Thus, the therapeutic modality of ACLF-HBV was determined according to the physician's decision, not with a planned protocol. This might lead to a bias in the prognosis group allocation and analysis. Also, there were missing data such as serial MELD scores and the type, duration of the antiviral agent used. Second, the number of patients was too small to fully analyze the effective prognosis predictors for two or three subgroups. Because this study was based on single center data, data collection from multi centers could provide more valuable results. Most enrolled patients were referred from other hospitals at different times in both FG and UFG. It is possible that the patients who were referred earlier may have had more opportunities to consider LT for survival. Although the exact onset timing of acute hepatic decompensation before transfer was not available from medical records, the mean actual hospitalization duration till death or LT was shorter in the Death group (20 days) than in the LT group (45 days).
In conclusion, it is important to assess the dynamic progression of clinical features (aggravation of hepatic encephalopathy (≥grade III), development of infection or SIRS) earlier as well as the initial clinical presentation (initial high MELD score ≥28, presence of moderate or severe ascites) to predict the prognosis of ACLF-HBV. Early consideration of LT is necessary for the patients with these risk factors. Furthermore, serial measurement of MELD scores to determine delta MELD score can be useful to predict unfavorable prognosis.
Abbreviations
ALT
alanine aminotransferase
AST
aspartate aminotransferase
CRRT
continuous renal replacement therapy
Gr
grade
HE
hepatic encephalopathy
HRS
hepatorenal syndrome
INR
international normalized ratio
LT
liver transplantation
MELD
model of end-stage liver disease
PT
prothrombin time
SBP
spontaneous bacterial peritonitis
SIRS
systemic inflammatory response syndrome
REFERENCES
1. Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology 2010;52:886-893. 20683932.


2. World Health Organization. Fact sheet N°204 : Hepatitis B. WHO web site; 2008 8. Accessed 2014]. http://www.who.int/mediacenter/factsheets/fs204/en/.
3. Seto WK, Lai CL, Yuen MF. Acute-on-chronic liver failure in chronic hepatitis B. J Gastroenterol Hepatol 2012;27:662-669. 22098452.


4. Sarin SK, Kumar A, Almeida JA, Chawla YK, Fan ST, Garg H, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int 2009;3:269-282. 19669378.



5. Lok AS, Lai CL. Acute exacerbations in Chinese patients with chronic hepatitis B virus (HBV) infection. Incidence, predisposing factors and etiology. J Hepatol 1990;10:29-34. 2307827.


6. Yuen MF, Sablon E, Hui CK, Li TM, Yuan HJ, Wong DK, et al. Prognostic factors in severe exacerbation of chronic hepatitis B. Clin Infect Dis 2003;36:979-984. 12684909.


7. Wlodzimirow KA, Eslami S, Abu-Hanna A, Nieuwoudt M, Chamuleau RA. A systematic review on prognostic indicators of acute on chronic liver failure and their predictive value for mortality. Liver Int 2013;33:40-52. 22429562.


8. Hwang SJ, Lee SD, Lu RH, Chan CY, Lai L, Co RL, et al. Comparison of three different hybridization assays in the quantitative measurement of serum hepatitis B virus DNA. J Virol Methods 1996;62:123-129. 9002070.


9. Polson J, Lee WM. American Association for the Study of Liver Disease. AASLD position paper: the management of acute liver failure. Hepatology 2005;41:1179-1197. 15841455.


10. Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology 2001;33:464-470. 11172350.


11. Bajaj JS, Wade JB, Sanyal AJ. Spectrum of neurocognitive impairment in cirrhosis: Implications for the assessment of hepatic encephalopathy. Hepatology 2009;50:2014-2021. 19787808.


12. Arroyo V, Ginès P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology 1996;23:164-176. 8550036.


13. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992;20:864-874. 1597042.

14. Yu JW, Sun LJ, Zhao YH, Li SC. Prediction value of model for end-stage liver disease scoring system on prognosis in patients with acute-on-chronic hepatitis B liver failure after plasma exchange and lamivudine treatment. J Gastroenterol Hepatol 2008;23:1242-1249. 18637053.


15. Zheng MH, Shi KQ, Fan YC, Li H, Ye C, Chen QQ, et al. A model to determine 3-month mortality risk in patients with acute-on-chronic hepatitis B liver failure. Clin Gastroenterol Hepatol 2011;9:351-356.e3. 21195790.


16. Sun QF, Ding JG, Xu DZ, Chen YP, Hong L, Ye ZY, et al. Prediction of the prognosis of patients with acute-on-chronic hepatitis B liver failure using the model for end-stage liver disease scoring system and a novel logistic regression model. J Viral Hepat 2009;16:464-470. 19413694.


17. Wong VW, Wong GL, Yiu KK, Chim AM, Chu SH, Chan HY, et al. Entecavir treatment in patients with severe acute exacerbation of chronic hepatitis B. J Hepatol 2011;54:236-242. 21030105.


18. Chen CH, Lin CL, Hu TH, Hung CH, Tseng PL, et al. Entecavir vs. lamivudine in chronic hepatitis B patients with severe acute exacerbation and hepatic decompensation. J Hepatol 2014;60:1127-1134. 24583247.


19. Cui YL, Yan F, Wang YB, Song XQ, Liu L, Lei XZ, et al. Nucleoside analogue can improve the long-term prognosis of patients with hepatitis B virus infection-associated acute on chronic liver failure. Dig Dis Sci 2010;55:2373-2380. 20512414.


20. Bajaj JS, O'Leary JG, Reddy KR, Wong F, Olson JC, Subramanian RM, et al. Second infections independently increase mortality in hospitalized patients with cirrhosis: the North American consortium for the study of end-stage liver disease (NACSELD) experience. Hepatology 2012;56:2328-2335. 22806618.



Figure 1
Study design for enrollment and classification of patients with hepatitis-B-related acute-on-chronic liver failure according to outcome.
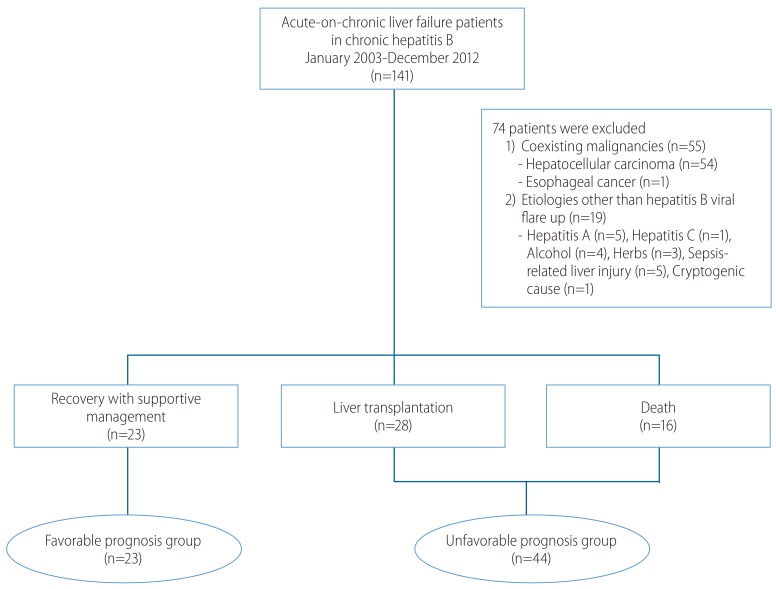
Figure 2
Repeated measurements of Model for End-Stage Liver Disease (MELD) scores at 3-day intervals in the Favorable prognosis group (A) and the Unfavorable prognosis group (B) revealed wide and overlapping distributions.
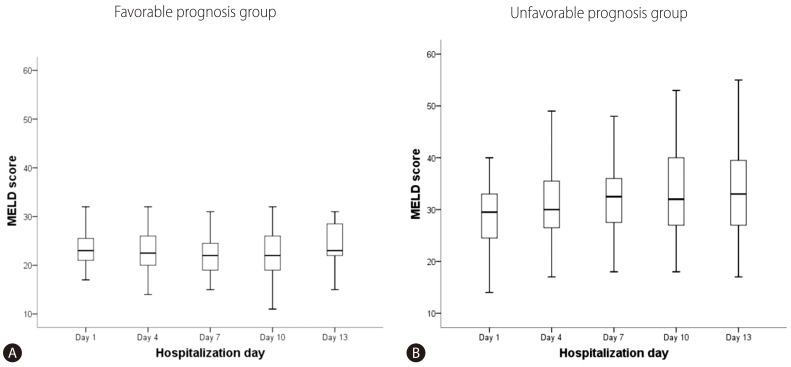
Figure 3
Comparison of the early dynamic changes in the predicted mean MELD scores between the Favorable prognosis group (lower line) and the Unfavorable prognosis group (upper line).
Table 1.
Baseline demographic, clinical, and biochemical characteristics of patients with acute-on-chronic liver failure according to different outcomes
Table 2.
Changes in clinical features during hospitalization
Table 3.
Risk factors for an unfavorable prognosis
| Univariable OR (95% CI) | P-value | Multivariable OR (95% CI) | P-value | |
|---|---|---|---|---|
| Gender | ||||
| Women | 1 | |||
| Men | 2 (0.3-2.95) | 0.166 | ||
| Age (years) | ||||
| <50 | 1 | |||
| ≥50 | 1.47 (0.71-6.5) | 0.220 | ||
| Liver cirrhosis | ||||
| Absence | 1 | |||
| Presence | 1.11 (0.29-4.28) | 0.876 | ||
| Platelet count (x103/mm3) | ||||
| ≥100 | 1 | |||
| <100 | 1.21 (0.44-3.32) | 0.717 | ||
| Serum albumin (g/dL) | ||||
| <3.0 | 1 | |||
| ≥3.0 | 0.94 (0.36-3.18) | 0.908 | ||
| AST (U/L) | ||||
| <400 | 1 | |||
| ≥400 | 0.97 (0.34-2.80) | 0.955 | ||
| ALT (U/L) | ||||
| <400 | 1 | |||
| ≥400 | 1.96 (0.65-5.94) | 0.233 | ||
| Total bilirubin (mg/dL) | ||||
| <25.0 | 1 | |||
| ≥25.0 | 4.74 (1.49-15.06) | 0.008 | ||
| Creatinine (mg/dL) | ||||
| <1.0 | ||||
| ≥1.0 | 1.96 (0.65-5.94) | 0.233 | ||
| MELD score at baseline | ||||
| <28 | 1 | |||
| ≥28 | 6.96 (2.16-22.44) | 0.001 | 6.64 (1.45-30.44) | 0.015 |
| HBeAg status | ||||
| Negative | 1 | |||
| Positive | 0.49 (0.15-1.6) | 0.225 | ||
| HBV DNA at baseline (IU/mL)* | ||||
| <2 x 106 | 1 | |||
| ≥2 x 106 | 2 (0.68-5.91) | 0.210 | ||
| Hepatic encephalopathy at baseline | ||||
| ≤Grade 2 | 1 | |||
| ≥Grade 3 | 2.7 (0.53-13.71) | 0.231 | ||
| Ascites at baseline | ||||
| Mild | 1 | |||
| Moderate ~ severe | 6.10 (2.01-18.47) | 0.001 | 6.71 (1.52-29.61) | 0.012 |
| Hepatorenal syndrome at baseline | ||||
| Absence | 1 | |||
| Presence | 9.23 (1.12-75.8) | 0.039 | ||
| Aggravation of encephalopathy | ||||
| Absence | 1 | |||
| Presence | 31.78 (3.92-257.48) | 0.001 | 15.41 (1.77-134.38) | 0.013 |
| SIRS | ||||
| Absence | 1 | |||
| Presence | 2.5 (0.86-7.28) | 0.092 | ||
| Infection including SBP | ||||
| Absence | 1 | |||
| Presence | 3.10 (1.03-9.35) | 0.044 | ||
| Infection excluding SBP | ||||
| Absence | 1 | |||
| Presence | 1.57 (0.71-17.39) | 0.105 | ||
| Nucleos (t)ide agent for treatment* | ||||
| Lamivudine and others | 1 | |||
| Entecavir | 1.66 (0.56-4.98) | 0.364 |
- TOOLS
-
METRICS

- Related articles
-
The fibrogenic process and the unleashing of acute-on-chronic liver failure2020 January;26(1)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



