| Clin Mol Hepatol > Volume 28(3); 2022 > Article |
|
ABSTRACT
ACKNOWLEDGMENTS
FOOTNOTES
Figure 1.
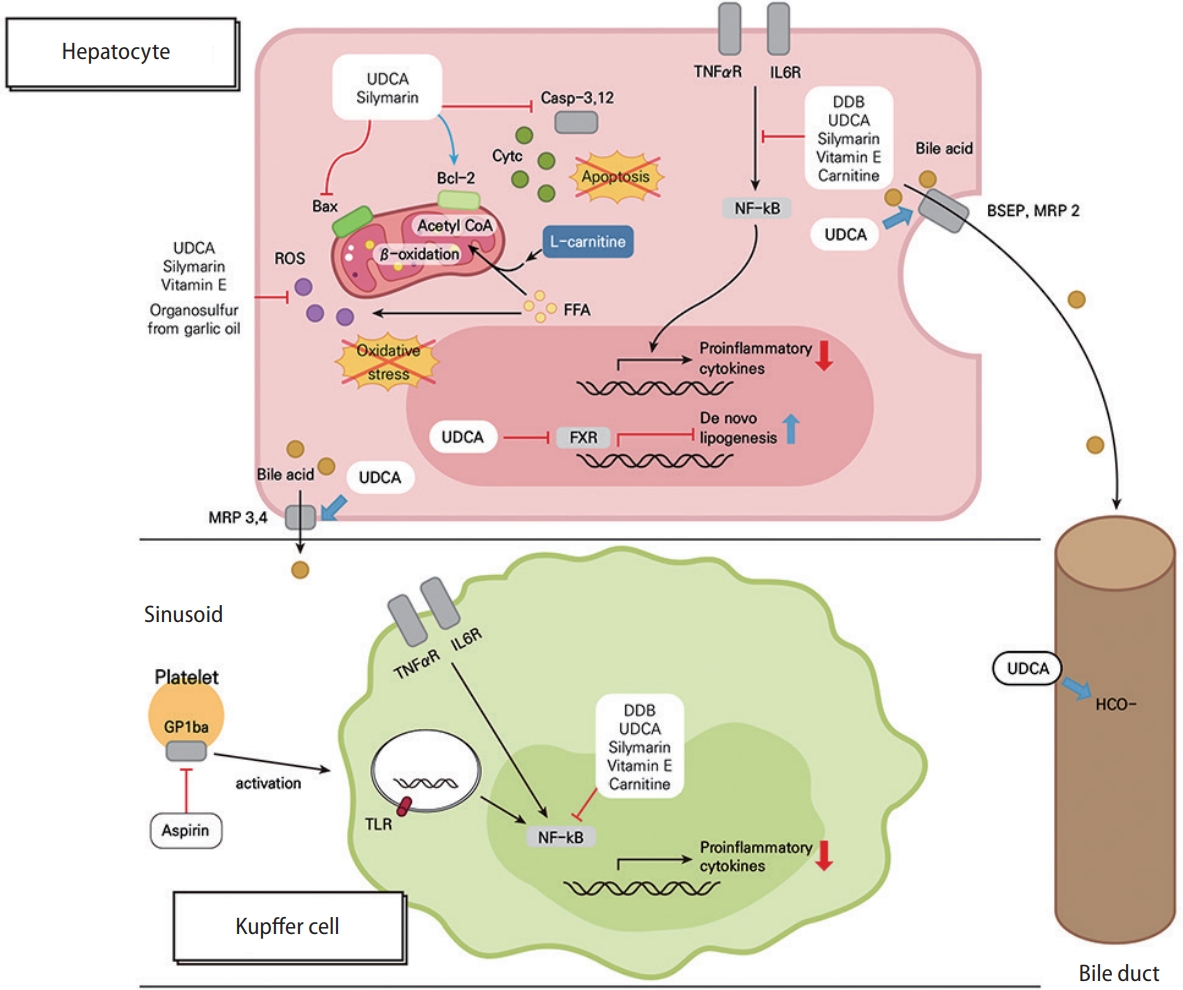
Figure 2.
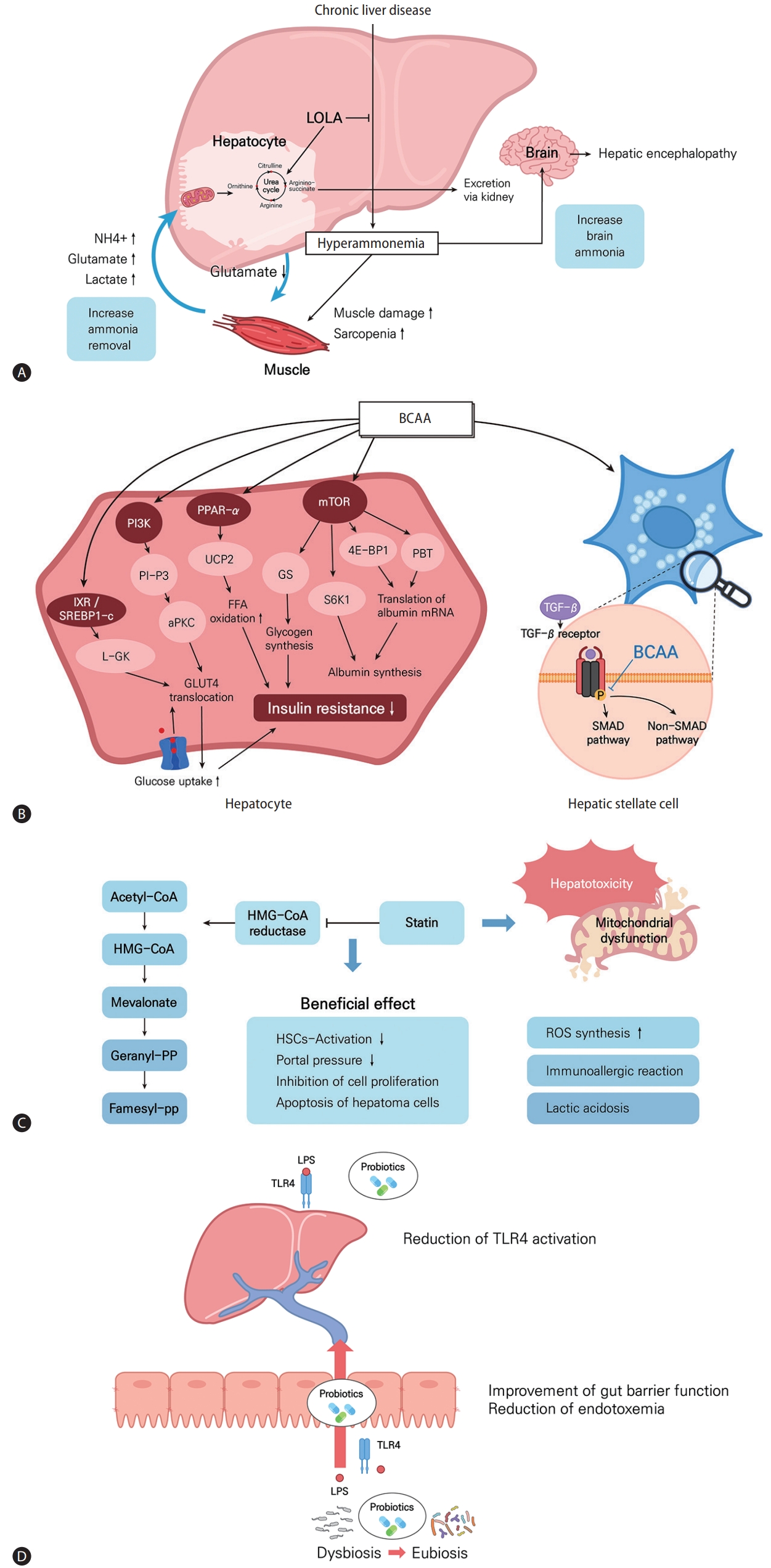
Figure 3.
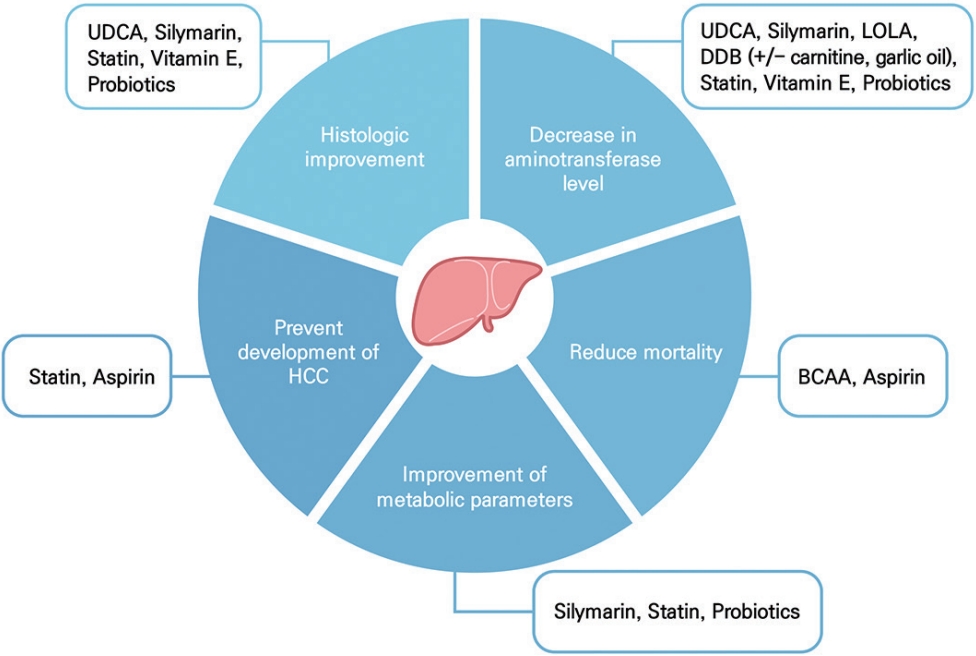
Table 1.
| Study | Etiology | Inclusion criteria | Intervention period | Arms (n) | Age (years) | Outcomes | Study design |
|---|---|---|---|---|---|---|---|
| Lindor et al. [8] (2004) | NAFLD | Biopsy-proven NASH with ALT >1.5×ULN | 2 years | UDCA 13–15 mg/kg/day (80) | 45.4±12.0 | · Changes in AST (mean, -21.7 U/L vs. -20.7 U/L; P=0.37) and ALT (mean, -32.7 U/L vs. -31.6 U/L; P=0.60) | RCT |
| Placebo (86) | 48.5±11.6 | · Changes in steatosis (mean, -0.6 vs. -0.3; P=0.41), inflammation (mean, 0.0 vs. -0.1; P=0.43), and fibrosis (mean, 0.0 vs. 0.0; P=0.50) stages | |||||
| Leuschner et al. [58] (2010) | NAFLD | Biopsy-proven NASH with ALT >1.5×ULN | 18 months | UDCA 23–28 mg/kg/day (94) | 41.45 (18–71) | · Difference in modified Brunt score (mean, -0.98 vs. -0.97; P=0.881) | RCT |
| Placebo (91) | 45.02 (18–73) | · Difference in NAS (mean, -1.22 vs. -1.03; P=0.355) | |||||
| Ratziu et al. [59] (2011) | NAFLD | Biopsy-proven NASH with ALT >50 IU/L | 12 months | UDCA 28–35 mg/kg/day (62) | 49.8±10.2 | · Changes in ALT (-28.3% vs. -1.6%; P<0.001) | RCT |
| Placebo (61) | 49.6±12.6 | · Proportion of ALT normalization (24.5% vs. 4.8%; P<0.003) | |||||
| · Changes in FibroTest values (median, -10.5% vs. +9.6%; P<0.006) | |||||||
| Traussnigg et al. [63] (2019) | NAFLD | ALT >0.8×ULN | 12 weeks | Nor-UDCA 1,500 mg/day (67) | 48.9±12.8 | · Changes in ALT (mean, -17.2 U/L vs. -7.0 U/L vs. +5.3 U/L;P<0.0001) | RCT |
| Nor-UDCA 500 mg/day (67) | 44.9±11.6 | · Proportion of ALT < 0.8×ULN (17.5% vs. 14.8% vs. 5.2%) | |||||
| Placebo (64) | 48.8±11.4 | · Reduction of hepatic fat fraction by MRS (-23.5% vs. +0.9% vs. -1.0%) | |||||
| Fabbri et al. [64] (2000) | CHC | Non-responders to IFN-α with ALT >1.5×ULN | 14 months | IFN-α + UDCA 600 mg/day followed by UDCA 600 mg/ day (53) | 52.6±1.8 | · Proportion of ALT normalization (38% vs. 12%; P<0.01) | RCT |
| IFN-α followed by placebo (50) | 45.8±1.8 | · Proportion of ALT relapse after withdrawal of IFN-α (55% vs. 100%; P<0.01) | |||||
| Boucher et al. [65] (2000) | CHC | Responders to IFN-α | 12 months | UDCA 10 mg/kg/day (54) | 41±15 | · Proportion of biochemical SR (30% vs. 46%; P=NS) | RCT |
| Placebo (53) | 43±15 | · Proportion of virological SR (22% vs. 32%; P=NS) | |||||
| · Post-treatment Knodell score (mean, 6.6 vs. 5.6; P=NS) | |||||||
| Omata et al. [66] (2007) | CHC | ALT >61 U/L | 24 weeks | UDCA 150 mg/day (195) | 58±12.2 | · Changes in ALT (-15.3% vs. -29.2% vs. -36.2%; P<0.001) | RCT |
| UDCA 600 mg/day (198) | 57.7±12.0 | · Changes in AST (-13.6% vs. -25.0% vs. -29.8%; P<0.001) | |||||
| UDCA 900 mg/day (193) | 59.8±10.1 | · Changes in GGT (-22.4% vs. -41.0% vs. -50.0%; P<0.001) |
Variables are expressed as median (interquartile range) or mean±standard deviation.
UDCA, ursodeoxycholic acid; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; ALT, alanine aminotransferase; ULN, upper limit of normal; AST, aspartate aminotransferase; RCT, randomized controlled trial; NAS, NAFLD activity score; nor-UDCA, norursodeoxycholic acid; MRS, magnetic resonance spectroscopy; CHC, chronic hepatitis C; IFN, interferon; SR, sustained response; NS, not significant.
Table 2.
| Study | Etiology | Inclusion criteria | Intervention period | Arms (n) | Age (years) | Outcomes | Study design |
|---|---|---|---|---|---|---|---|
| Navarro et al. [9] (2019) | NAFLD | NAS ≥4 without cirrhosis | 48–50 weeks | Silymarin 1,260 mg/day (26) | 47.3 (10.8) | · Improvement ≥2 in NAS (19% vs. 15% vs. 12%; P=0.79) | RCT |
| Silymarin 2,100 mg/day (27) | 48.2 (11.4) | · Normalized ALT level (8% vs. 25% vs. 5%; P=0.08) | |||||
| Placebo (25) | 49.5 (10.9) | · Improved fibrosis stage (12% vs. 26% vs. 28%; P=0.30) | |||||
| Wha Kheong et al. [31] (2017) | NAFLD | NAS ≥4 | 48 weeks | Silymarin 2,100 mg/day (49) | 49.6±12.7 | · Decrease ≥30% in NAS (32.7% vs. 26.0%; P=0.467) | RCT |
| Placebo (50) | 50.1±10.2 | · Reductions in fibrosis ≥1 stage (22.4% vs. 6.0%; P=0.023) | |||||
| · Change in triglyceride level (mean, -0.2 vs. 0.04 mmol/L; P=0.017) | |||||||
| Anushiravani et al. [87] (2019) | NAFLD | Grade ≥2 steatosis in ultrasonography | 3 months | LSM (30) | 47.0±9.1 | · Changes in AST (mean, -0.9 vs. -8.3 U/mL; P<0.001) and ALT (mean, -0.6 vs. -9.3 U/mL; P<0.001) levels | RCT |
| LSM + silymarin 140 mg/day (30) | · Changes in WC (mean, -1.2 vs. -0.3 cm; P<0.001) | ||||||
| Solhi et al. [85] (2014) | NAFLD | AST and ALT >1.2×ULN | 8 weeks | LSM + silymarin 210 mg/day (33) | 43.6±8.3 | · Changes in AST (mean, 62.8 to 30.5 vs. 70.4 to 36.2; P=0.038) and ALT (mean, 91.3 to 38.4 vs. 84.6 to 52.3; P=0.026) levels (U/L) | RCT |
| LSM + placebo (31) | 9.4±10.5 | ||||||
| Hajiaghamohammadi et al. [28] (2012) | NAFLD | 8 weeks | Pioglitazone 15 mg/day (22) | 33.4±6.6 | · Changes in AST (mean, 55.0 to 37.59 vs. 54.86 to 42.5 vs. 56 to 37.77; P=0.003) and ALT (mean, 77.45 to 52.27 vs. 78.36 to 60.95 vs. 78.73 to 53.05; P<0.005) levels (U/L) | RCT | |
| Metformin 500 mg/day (22) | 32.5±6.5 | ||||||
| Silymarin 140 mg/day (22) | 33.5±6.3 | · Significant reductions in mean levels of fasting glucose, triglyceride, total cholesterol, and insulin and HOMA-IR in all groups (P<0.01) | |||||
| Hashemi et al. [86] (2009) | NAFLD | AST and ALT >1.2×ULN or biopsy-proven NASH | 6 months | Silymarin 280 mg/day (50) | 39.28±11.11 | · ALT (52% vs. 18%; P=0.001) and AST (62% vs. 20%; P=0.0001) level normalization | RCT |
| Placebo (50) | 39.0±10.70 | ||||||
| Sorrentino et al. [90] (2015) | NAFLD | WC > 94 cm in men or >80 cm in women, triglyceride >150 mg/dL, and fasting glucose >100 mg/dL | 90 days | LSM + silymarin 250 mg/day + vitamin E 60 IU/day (43) | 56.63±12.79 | · Change in hepatic steatosis index (mean, -1.85 vs. -0.19; P=0.0134) | Prospective cohort study |
| LSM (35) | 55.40±13.63 | · Changes in BMI (mean, -0.71 vs. -0.004 kg/m2; P=0.022) and WC (mean, -4.81 vs. -1.78 cm; P=0.028) | |||||
| Stiuso et al. [88] (2014) | NAFLD | Biopsy-proven NASH | 12 months | Low levels of TBARS: Silymarin 188 mg/day + phosphatidyl choline 388 mg/day + vitamin E acetate 50% 178.56 mg/day (11) | 40.8±10.3 | · Proportion of change in NAS (-29%; NS), portal inflammation (-25%; NS), and fibrosis (-50%; P=0.01) | Retrospective cohort study |
| · Proportion of change in AST (-33%; P=0.05), ALT (-14%; NS), and insulin (-8%; NS) levels and HOMA-IR (-11%; NS) | |||||||
| High levels of TBARS: silymarin 188 mg/day + phosphatidyl choline 388 mg/day + vitamin E acetate 50% 178.56 mg/day (19) | · Proportion of change in NAS (-70%, P=0.001), portal inflammation (-58%; P=0.001), and fibrosis (-60%; P=0.001) | ||||||
| · Proportion of change in AST (-42%; P=0.01), ALT (-31%; P=0.05), and insulin (-40%; P=0.001) levels and HOMA-IR (-42%; P=0.001) | |||||||
| Fried et al. [91] (2012) | HCV | ALT ≥65 U/L who were unsuccessfully treated with IFN | 24 weeks | Silymarin 1,260 mg/day (50) | 54.0 (52.0–57.0) | · Proportion of ALT ≤45 U/L (4.0% vs. 3.8% vs. 1.9%; P=0.80), at least 50% ALT decline and ALT <65 U/L at week 24 (2.0% vs. 3.8% vs. 3.8%; P=0.83) | RCT |
| Silymarin 2,100 mg/day (52) | 54.0 (48.0–58.0) | ||||||
| Placebo (52) | 56.0 (51.5–59.5) | · Changes in ALT (mean, -14.4 vs. -11.3 vs. -4.3 U/L; P=0.75) and HCV RNA (mean, -0.03 vs. 0.04 vs. 0.07 log10 IU/L; P=0.54) levels | |||||
| Yakoot and Salem [92] (2012) | HCV | Genotype 4, IFN-naïve or relapsers/nonresponders to IFN or combined therapy | 6 months | Silymarin 420 mg/day (29) | 48±12 | · ETR (3.4% vs. 13.3%; P=0.12) | RCT |
| Spirulina 1,500 mg/day (30) | 47±12 | · 3 months virological response (0% vs. 3.3%; P=0.22) | |||||
| · Reduction in ALT level (mean, -6.8 vs. -23.7 IU/L; P=0.006) | |||||||
| Tanamly et al. [94] (2004) | HCV | Presence of HCV RNA | 12 months | Silymarin 373.5 mg/day (68) | 44.1 | · All physical and mental health variables (SF-36 questionnaire) were improved in both groups. | RCT |
| Multivitamin (71) | · Persistent HCV RNA (97.1% vs. 95.8%; P=0.684) and ALT >35 IU/L (13.0% vs. 14.3%; NS) | ||||||
| Ferenci et al. [95] (2008) | HCV | Nonresponders to full-dose PegIFN/RBV | 14 days | Silymarin iv 5 (3), 10 (3), 15 (5), 20 (9) mg/kg + 24 weeks PegIFNα-2a 180 µg/week + ribavirin 1–1.2 g/day from day 8 | 52.7±12.8 | · Dose-dependent HCV RNA decrease (log drop after 14 days: mean, 1.63 [5 mg/kg], 4.16 [10 mg/kg], 3.69 [15 mg/kg], and 4.85 [20 mg/kg]; P<0.001) | Prospective cohort study |
| · Undetectable HCV RNA in 7 patients on 15 or 20 mg/kg of silymarin at week 12 |
Variables are expressed as median (interquartile range) or mean±standard deviation.
NAFLD, nonalcoholic fatty liver disease; NAS, NAFLD activity score; ALT, alanine aminotransferase; RCT, randomized controlled trial; LSM, lifestyle modification; AST, aspartate aminotransferase; WC, waist circumference; ULN, upper limit of normal; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; NASH, nonalcoholic steatohepatitis; BMI, body mass index; TBARS, thiobarbituric acid-reactive species; NS, not significant; HCV, hepatitis C virus; IFN, interferon; ETR, end-treatment response; PegIFN/RBV, pegylated interferon and ribavirin.
Table 3.
| Study | Etiology | Inclusion criteria | Intervention period | Arms (n) | Age (years) | Outcomes | Study design |
|---|---|---|---|---|---|---|---|
| Lee et al. [10] (2014) | CLD | (1) Persistent ALT ≥1.5×ULN more than once during the previous 6 months; (2) ALT ≥1.5×ULN at enrollment | 24 weeks | DDB 750 mg/day (67) | 51 (20–72) | ALT normalization (≤40 IU/L) (80.0% vs. 34.8%; P<0.001) | RCT; double blind, active- controlled |
| Kang et al. [108] (2001) | CLD | (1) Biopsy-proven chronic hepatitis or (2) abnormal AST/ALT for >6 months; no evidence of viral hepatitis B or C | 8 weeks | High-dose group: DDB 150 mg + carnitine/orotate complex 900 mg/day (33) | 49.03±9.74 | ALT normalization (88.5% vs. 54.6% vs.44.4%; P=0.0027) | RCT; double blind, phase II |
| Low-dose group: DDB 150 mg + carnitine/orotate complex 600 mg/day (30) | 41.58±11.73 | ||||||
| DDB 150 mg/day (32) | 44.00±11.55 | ||||||
| Park et al. [109] (2001) | CLD | (1) Biopsy- proven chronic hepatitis or (2) ALT elevation (≥1.5×ULN) more than twice during the previous 6 months; no evidence of viral hepatitis B or C | 8 weeks | High-dose group: DDB 150 mg + carnitine/orotate complex 900 mg/day (53) | 44.57±11.49 | ALT normalization (81.13% vs. 67.35% vs. 64.54%; P=0.0407) | RCT; double blind, phase III |
| Low-dose group: DDB 100 mg + carnitine/orotate complex 600 mg/day (48) | 43.24±13.01 | ||||||
| DDB 100 mg/day (52) | 45.63±13.75 | ||||||
| Kim et al. [110] (2014) | CLD | (1) Abnormal ALT or AST in previous 6 months, (2) sonographical findings of chronic hepatitis or fatty liver, or (3) history of being treated for chronic hepatitis for >30 days | 12 weeks | DDB + garlic oil 960 mg/day (100) | 44 (20–79) | ALT normalization (≤40 IU/L) (89% vs. 18.6% vs. 22.9%; P<0.001) | RCT;double blind, placebo- andactive- controlled, phaseIV |
| Silymarin 1,018 mg/day (102) | 49 (20–75) | ||||||
| Placebo (35) | 44 (24–77) | ||||||
| Hong et al. [111] (2014) | NAFLD | Combined with impaired fasting glucose metabolism | 12 weeks | Metformin 750 mg/day and DDB-carnitine orotate complex 900 mg/day (26) | 51.5±9.4 | Decrement of ALT level (mean, 51.5 vs. 16.7; P=0.001) | RCT; double blind, placebo- controlled |
| Metformin 750 mg/day and placebo (26) | 52.0±9.6 | ||||||
| Bae et al. [112] (2015) | NAFLD | Combined with type 2 diabetes | 12 weeks | DDB-carnitine orotate complex 824 mg/day (39) | 50.6±9.3 | ALT normalization (<30 IU/L in men or <19 IU/L in women) (89.7% vs. 17.9%; P<0.001) | RCT; double blind, placebo- controlled |
| Placebo (39) | 52.0±9.4 | ||||||
| Jun et al. [113] (2013) | HBV | (1) ALT ≥80 IU/L, (2) ALT < ULN×10, (3) treatment- naïve, and (4) HBV >105 copies/ mL in case of HBeAg-positive result or HBV >104 copies/ mL in case of HBeAg-negative result | 12 months | Entecavir 0.5 mg/day and DDB- carnitine orotate 2,472 mg/ day complex (67) | 43.0 ± 9.8 | ALT normalization (<40 U/L) (100% vs. 85.7%; P=0.0019) | RCT |
| Entecavir 0.5 mg/day (63) | 44.9±10.0 |
Variables are expressed as median (interquartile range) or mean±standard deviation.
DDB, dimethyl-4,4’-dimethoxy-5,6,5’,6’-dimethylenedixoybiphenyl-2,2’-dicarboxylate; CLD, chronic liver disease; ALT, alanine aminotransferase; ULN, upper limit of normal; RCT, randomized controlled trial; AST, aspartate aminotransferase; NAFLD, nonalcoholic fatty liver disease; HBV, hepatitis virus; HBeAg, hepatitis B e antigen.
Table 4.
| Study | Etiology | Inclusion criteria | Intervention period | Arms (n) | Age (years) | Outcome | Study design |
|---|---|---|---|---|---|---|---|
| Muto et al. [11] (2005) | Mixed (mainly HCV) | Decompensated cirrhosis | 2 years | BCAA (314) | 62±8 | Higher/longer event-free survival in BCAA group (death, varix rupture, HCC, hepatic failure) (HR, 0.67; 95% CI, 0.49–0.93, P=0.015) | RCT |
| Control (308) | 61±9 | ||||||
| Marchesini et al. [144] (2003) | Mixed (mainly viral) | Advanced cirrhosis (CTP B or C) | 1 year | BCAA (59) | 59±1 | Event (death, varix rupture, HCC, hepatic failure) (15.5% vs. 32.1% vs. 27.1%; P=0.037) | RCT |
| Lactoalbumin (56) | 60±1 | ||||||
| Maltodextrins (59) | 59±1 | ||||||
| Ichikawa et al. [145] (2010) | Mixed (mainly viral) | Liver cirrhosis | 8 weeks | BCAA (12) | 66.2±8.2 | Change in ESS (mean, -5.5 vs. 1.2; P<0.05) | RCT |
| Control (9) | 67.4±9.8 | ||||||
| Yamamoto et al. [154] (2005) | Mixed (mainly viral) | Liver cirrhosis | 1 hour | BCAA (16) | 63±8 | Change in cerebral blood flow (PET) (mean, 0.81 vs. 0.75; P<0.05) | RCT |
| Control (13) | 62±9 | ||||||
| Kawamura et al. [138] (2009) | Mixed (mainly HCV) | Liver cirrhosis with CTP class A | 1 year | BCAA (27) | 62.7±10.08 | Cirrhosis-related complications (HCC, ascites, varix, HE) (14.8% vs. 30.4%; P=0.043) | RCT |
| Control (23) | 62.3±7.3 | ||||||
| Les et al. [32] (2011) | Mixed (mainly HCV, alcohol) | Liver cirrhosis with episode of HE within 2 months | 56 weeks | BCAA (21) | 64.1±10.4 | Recurrence of HE (47% vs. 34%; P=0.274) | RCT |
| Control, maltodextrin (27) | 62.5±10.4 | ||||||
| Nakaya et al. [139] (2007) | HCV | Liver cirrhosis | 3 months | BCAA (19) | 67±9 | Change in albumin level (mean, 3.2 vs. 3.0; P<0.05) | RCT |
| Control (19) | 67±8 | ||||||
| Takeshita et al. [29] (2012) | HCV | HCV with insulin resistance | 24 weeks | BCAA (14) | 58.6±2.9 | HOMA-IR after treatment (mean, 4.5 vs. 5.3; P=0.047) | RCT |
| Control (13) | 64.2±3.0 | ||||||
| Koreeda et al. [149] (2011) | Mixed (mainly HCV) | Liver cirrhosis | 6 months | BCAA (17) | 68±10 | Change in Rmax (mean, 0.23 to 0.25; P=0.059) | Prospective cohort study |
| Park et al. [142] (2020) | Mixed (mainly alcohol) | Liver cirrhosis with CTP score of 8–10 points | 6 months | BCAA (63) | 60±10 | Higher/longer event-free survival in BCAA group (death, varix rupture, HCC, hepatic failure) (HR, 0.38; 95% CI, 0.22–0.68, P<0.001) | Prospective cohort study |
| Control (61) | 58±11 | ||||||
| Park et al. [143] (2017) | Mixed (mainly HBV, alcohol) | Liver cirrhosis with CTP score of 8–10 points | 6 months | BCAA (166) | 59±11 | Cirrhotic complication-free survival (median, 19.3 vs. 19.2 months; P=0.973) | Retrospective cohort study |
| Control (141) | 60±9 | ||||||
| Hanai et al. [277] (2015) | Mixed (mainly HCV) | Liver cirrhosis patients who were not transplantation candidates | 1 year | BCAA (94) | 64 (28–91) | Higher/longer overall survival in BCAA group (log-rank test P=0.02) | Retrospective cohort study |
| Control (36) | 66 (33–84) | ||||||
| Hanai et al. [148] (2020) | Mixed (mainly HCV, alcohol) | Liver cirrhosis | NA | BCAA (87) | 69 (59–74) | Lower mortality in BCAA group (HR, 0.57; 95% CI, 0.33–0.99, P=0.046) | Retrospective cohort study |
| Control (436) | 66 (55–74) |
Variables are expressed as median (interquartile range) or mean±standard deviation.
BCAA, branched chain amino acid; HCV, hepatitis C virus; HCC, hepatocellular carcinoma; HR, hazard ratio; CI, confidence interval; RCT, randomized controlled trial; CTP, Child-Turcotte-Pugh; ESS, Epworth Sleepiness Scale; PET, positron emission tomography; HE, hepatic encephalopathy; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; HBV, hepatitis B virus.
Table 5.
| Study | Etiology | Inclusion criteria | Intervention period | Arms (n) | Age (years) | Outcomes | Study design |
|---|---|---|---|---|---|---|---|
| Nelson et al. [12] (2009) | NAFLD | Biopsy-proven NASH | 12 months | Simvastatin 40 mg/day (10) | 52.6±8.6 | · Necroinflammatory activity (mean, 1.4 vs. 1.0; P>0.05) | RCT |
| Control (6) | 52.5±13.0 | · Fibrosis stage (mean, 1.50 vs. 1.0; P>0.05) | |||||
| Dongiovanni et al. [165] (2015) | NAFLD | Patients who underwent liver biopsy for suspected NASH | ≥6 months | Statin (107) | 53±10 | · Lower presence of steatosis (OR, 0.09; 95% CI, 0.01–0.32; P=0.004), NASH (OR, 0.25; 95% CI, 0.13–0.47; P<0.001), F2–F4 fibrosis (OR, 0.42; 95% CI, 0.20–0.80; P=0.017)* in statin group | Cross-sectional study |
| Control (1,094) | 41±16 | ||||||
| Nascimbeni et al. [166] (2016) | NAFLD | Biopsy-proven NAFLD with type 2 DM | NA | Statin (154) | 55 (48–61) | · Lower presence of NASH (OR, 0.57; 95% CI, 0.32–1.01; P=0.055) and significant fibrosis (OR, 0.47; 95% CI, 0.26–0.84; P<0.011) in statin group | Cross-sectional study |
| Control (192) | 52 (42–58) | ||||||
| Ekstedt et al. [167] (2007) | NAFLD | Biopsy-proven NAFLD with elevated ALT and/or AST >41 U/L and/ or elevated ALP >106 U/L | 10.3–16.3 years | Statin (17) | 48.7±9.1 | · Significant reduction of liver steatosis in statin group (20.4% to 11.1%, P=0.001) | Retrospective cohort study |
| Control (51) | 46.3±11.8 | ||||||
| Rallidis et al. [168] (2004) | NAFLD | Biopsy-proven NASH and abnormal liver enzyme | 6 months | Pravastatin 20 mg/day (5) | 40±8 | · Improvement in the grade of inflammation (75%) and steatosis (25%) | Prospective cohort study |
| Hyogo et al. [169] (2008) | NAFLD | Biopsy-proven NASH with lipidemia | 24 months | Atorvastatin 10 mg/day (31) | 52.5 (27–68) | · Significant improvement of steatosis grade (1.6 to 0.8; P<0.001) and NAS (4.1 to 2.9; P<0.001) | Prospective cohort study |
| Hyogo et al. [172] (2011) | NAFLD | Biopsy-proven NASH and hyperlipidemia | 12 months | Pitavastatin 2 mg/day (20) | 50.6 (25–75) | · Change of NAS (6.7 to 6.3) and fibrosis stage (2.3 to 2.1) | Prospective cohort study |
| Nakahara et al. [170] (2012) | NAFLD | Biopsy-proven NASH and hyperlipidemia | 24 months | Rosuvastatin 2.5 mg/day (19) | 46.3 (20–65) | · Change of NAS (3.89 to 3.44) and fibrosis stage (2.33 to 2.00) | Prospective cohort study |
| Kargiotis et al. [171] (2015) | NAFLD | Biopsy-proven NASH and metabolic syndrome and lipidemia | 12 months | Rosuvastatin 10 mg/day (20) | 40.5±5.6 | · Complete resolution of NASH (95%) | Prospective cohort study |
| Lee et al. [278] (2021) | NAFLD | Age ≥20 years who participated the NHIS physical health examination | 72 months | NAFLD (164,856) | 41.4±12.4 | · Reduced risk of NAFLD development in statin group (adjusted OR 0.66; 95% CI, 0.65–0.67) | Retrospective cohort study |
| Control (824,280) | 41.4±12.4 | ||||||
| Zou et al. [175] (2022) | NAFLD | Diabetes or obesity and ICD- 10 codes (K76.0 and K758.1) | 1.92 years | Statin (73,385) | 58.0±12.4 | · Lower risk of HCC development in statin group (HR, 0.47; 95% CI, 0.36–0.60; P<0.001) | Retrospective cohort study |
| Control (199,046) | 50.0±14.9 | ||||||
| Avins et al. [176] (2008) | Mixed | Patients with evidence of liver disease showing elevated AST or ALT levels or diagnosis of liver disease | 28.8 months (12.1–58.2) | Lovastatin (13,491) | 53.9±11.4 | · Lower incidence of liver function test abnormalities in statin group (incident RR 0.28; 95% CI, 0.12–0.55; P=NA) | Retrospective cohort study |
| Control (79,615) | 47.5±13.6 | ||||||
| Hsiang et al. [177] (2015) | HBV | 1.6 years | Statin (1,176) | 58.7±12.4 | · Lower HCC development in statin group (subHR 0.68; 95% CI, 0.48–0.97; P=0.033) | Retrospective cohort study | |
| 4.9 years | Control (52,337) | 37.6±14.1 | |||||
| Huang et al. [178] (2016) | HBV | 4.71±3.21 | Statin (22,544) | 52.87±11.51 | · Lower incidence of cirrhosis (RR, 0.433; 95% CI, 0.344–0.515; P<0.001) and decompensated cirrhosis (RR, 0.468; 95% CI, 0.344–0.637; P<0.001) in stain group | Retrospective cohort study | |
| 4.57±3.20 years | Control (215,802) | 39.73±13.14 | |||||
| Butt et al. [273] (2015) | HCV | Received HCV treatment ≥14 days | >24 months | Statin (3,347) | 53 (49–56) | · Higher SVR rate (OR, 1.44; 95% CI, 1.29–1.61; P<0.0001) in statin group | Retrospective cohort study |
| Control (3,901) | 52 (48–56) | · Cirrhosis development (17.3% vs. 25.2%; P<0.001) | |||||
| · HCC incidence (1.2% vs. 2.6%, P<0.01) | |||||||
| Simon et al. [179] (2015) | HCV | Previous non- response to standard interferon therapies and advanced hepatic fibrosis on liver biopsy | 3.5 years | Statin (29) | 54.2±7.2 | · Lower risk of fibrosis progression in statin group (unadjusted HR, 0.32; 95% CI, 0.10–0.97; P=0.048; adjusted HR, 0.31; 95% CI, 0.10–0.97; P=0.044) | Retrospective cohort study |
| Control (514) | |||||||
| Yang et. el. [180] (2015) | HCV | 2,874,031.7 personyears | Statin (29,204) | Only distribution available | · Incidence rate of cirrhosis (445.5/100,000 person-years vs. 1311.2/100,000 personyears) | Retrospective cohort study | |
| Control (197,652) | |||||||
| Mohanty et al. [181] (2016) | HCV | 2.6 years | Statin (1,323) | 56 (52–60) | · Lower risk of decompensation (HR, 0.22; 95% CI, 0.17–0.28) and death (HR, 0.39; 95% CI, 0.34–0.44) before matching in statin group | Retrospective cohort study | |
| 1.9 years | Control (12,522) | 54 (50–58) | · Lower risk of decompensation (HR, 0.55; 95% CI, 0.39–0.77 and death (HR, 0.56; 95% CI, 0.46–0.69) after matching in statin group | ||||
| Abraldes et al. [182] (2009) | Mixed (mainly alcohol) | Liver cirrhosis patients with severe portal HTN defined as HVPG of ≥12 mmHg | 30±4 days | Simvastatin (28) | 58±10 | · Change in HVPG (mean, -8.3% vs. -1.6%; P=0.041) | RCT |
| Control (27) | 56±10 | ||||||
| Pollo-Flores et al. [183] (2015) | Mixed (mainly HCV) | Cirrhosis with portal HTN | 3 months | Simvastatin (14) | 56.5 (IQR, 8.7) | · Decreased HVPG of at least 20% from baseline or to ≤12 mmHg) (55% vs. 0%; P=0.03) | RCT |
| Control (20) | 58.5 (IQR, 13.5) | ||||||
| Abraldes et al. [184] (2016) | Mixed (mainly alcohol) | Diagnosis of liver cirrhosis, and variceal bleeding within the previous 5–10 days, and plan to start standard prophylactic treatment for variceal bleeding | 371 days | Simvastatin (69) | 57.42±11.31 | · Higher survival (HR, 0.387; 95% CI, 0.152–0.986; P=0.030) in statin group | RCT |
| 382 days | Control (78) | 57.62±10.59 | · Lower rebleeding risk (HR, 0.858; 95% CI, 0.455–1.620, P=0.583) in statin group | ||||
| Kumar et al. [185] (2014) | Mixed (mainly HCV) | Biopsy-proven liver cirrhosis on statin therapy at biopsy and for ≥3 months after biopsy | 36 months | Statin (81) | 59.79±10.91 | · Lower mortality (HR, 0.53; 95% CI, 0.334–0.856; P=0.01) in statin group | Retrospective cohort study |
| 30 months | Control (162) | 59.64±10.60 | · Lowe risk of decompensation (HR, 0.58, 0.34–0.98; P=0.04) in statin group |
Variables are expressed as median (interquartile range) or mean±standard deviation.
NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; RCT, randomized controlled trial; OR, odds ratio; CI, confidence interval; DM, diabetes mellitus; NA, not available; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; NAS, NAFLD activity score; NHIS, National Health Interview Survey; ICD-10, International Classification of Diseases; HCC, hepatocellular carcinoma; HR, hazard ratio; RR, risk ratio; HBV, hepatitis B virus; HCV, hepatitis C virus; SVR, sustained viral response; HTN, hypertension; HVPG, hepatic venous pressure gradient; IQR, interquartile range.
Table 6.
| Study | Etiology | Inclusion criteria | Treatment | Intervention period | Arms (n) | Age (years) | Outcomes | Study design |
|---|---|---|---|---|---|---|---|---|
| Wong et al. [202] (2013) | NAFLD | Biopsy-proven NASH | Mixture of L. plantarum, L. delbrueckii, L. acidophilus, L. rhamnosus, and B. bifidum | 6 months | Probiotics (10) | 42±9 | · Decreased liver fat contents in probiotics (22.6% to 14.9%; P=0.034) | RCT |
| Usual care (10) | 55±9 | · Decreased AST level in probiotics (mean, 83.3 to 46.1; P=0.008) | ||||||
| Shavakhi et al. [205] (2013) | NAFLD | Biopsy-proven NASH on metformin | Lactobacillus, Bifidobacterium, Streptococcus | 6 months | Probiotics + metformin (31) | 41.5±12.7 | · Decreased ALT and AST levels (mean, 133.7 to 45.2 vs. 123.1 to 44.2; P<0.001) | RCT |
| Placebo + metformin (32) | 55±9 | · Reduction in grade of hepatic steatosis measured by US | ||||||
| Nabavi et al. [203] (2014) | NAFLD | NAFLD on US | L. acidophilus and B. lactis | 8 weeks | Probiotic yoghurt (36) | 42.74±8.72 | · Lower AST, ALT, total cholesterol, and LDL-cholesterol levels after treatment in probiotic yogurt groups than control (P<0.05) | RCT |
| No probiotics (15) | 44.05±8.14 | |||||||
| Abdel Monem [201] (2017) | NAFLD | Biopsy-proven NASH | L. acidophilus | 1 month | Probiotics (15) | 44.20±5.51 | · Decreased ALT level in probiotics (mean, 83.3 to 46.1; P<0.001) | RCT |
| No probiotics (15) | 44.33±5.62 | · Decreased AST level in probiotics (mean, 57.1 to 38.2; P=0.03) | ||||||
| Manzhalii et al. [210] (2017) | NAFLD | NASH fed a low- fat/low-calorie diet | L. casei, L. rhamnosus, L. bulgaris, B. longum, S. thermophilus and oligofructose | 12 weeks | Probiotic cocktail (38) | 44.3±1.5 | · Greater reduction in AST and ALT levels in synbiotics than placebo (P<0.05) | RCT |
| No probiotics (37) | 43.5±1.3 | · Greater reduction in the fibrosis score by TE in synbiotics than placebo (P<0.05) | ||||||
| Kobyliak et al. [204] (2018) | NAFLD | Diabetes | Bifidobacterium, Lactobacillus, Lactococcus, Propionibacterium | 8 weeks | Probiotics (30) | 53.4±9.55 | · Decreased AST and GGT levels in probiotics (P<0.05) | RCT |
| Placebo (28) | 57.3±10.5 | · Decreased fatty liver index in probiotics (84.33 to 78.73; P<0.001) | ||||||
| Ahn et al. [207] (2019) | NAFLD | Obesity and liver fat >5% on proton density fat fraction | Lactobacillus, Pediococcus, Bifidobacterium | 12 weeks | Probiotics (30) | 41.7±12.49 | · Decreased liver fat contents (mean 16.3% to 14.1%; P=0.032) | RCT |
| Placebo (35) | 44.71±13.31 | · Greater reduction in the triglyceride level in probiotics than placebo (P=0.003) | ||||||
| Duseja et al. [206] (2019) | NAFLD | Biopsy-proven NAFLD | Lactobacillus, Bifidobacterium, Streptococcus | 1 year | Oral multistrain probiotic (19) | 38±10 | · Greater reduction in ALT level in probiotics than placebo (P=0.046) | RCT |
| Placebo (20) | 33±6 | · Greater reduction in the NAS and hepatic fibrosis in probiotics than placebo (P<0.05) | ||||||
| Malaguarnera et al. [30] (2012) | NAFLD | Abnormal serum aminotransferase levels | B. longum and oligofructose | 24 weeks | Synbiotics + LSM (33) | 46.9±5.4 | · Lower AST and LDL-cholesterol levels after treatment in synbiotics than placebo (P<0.05) | RCT |
| Placebo + LSM (33) | 46.7±5.7 | · Greater reduction in HOMA-IR and NASH activity score in synbiotics than placebo (P<0.05) | ||||||
| Eslamparast et al. [13] (2014) | NAFLD | NAFLD on US with ALT >60 IU/L for 6 months | Combination of L. casei, L. rhamnosus, S. thermophilus, B. breve, L. acidophilus, B. longum, L. bulgaricus and oligofructose | 28 weeks | Synbiotic capsule (26) | 46.35±8.8 | · Lower AST, ALT and GGT levels after treatment in synbiotics than placebo (P<0.001) | RCT |
| Placebo (26) | 45.69±9.5 | · Greater reduction in the fibrosis score by TE (mean, 9.36 to 6.38 vs. 7.92 to 7.16; P<0.001) | ||||||
| Asgharian et al. [219] (2016) | NAFLD | Combination of L. casei, L. rhamnosus, S. thermophilus, B. breve, L. acidophilus, B. longum, L. bulgaricus and oligofructose | 8 weeks | Synbiotics (40) | 46.57±1.7 | · Decreased grade of steatosis on US in synbiotics (P<0.005) | RCT | |
| Placebo (40) | 47.78±1.7 | |||||||
| Mofidi et al. [208] (2017) | NAFLD | BMI ≤25 | Combination of L. casei, L. rhamnosus, S. thermophilus, B. breve, L. acidophilus, B. longum, L. bulgaricus and oligofructose | 28 weeks | Synbiotics (21) | 40.09±11.44 | · Greater reduction in AST and fasting glucose levels in synbiotics than placebo (P<0.05) | RCT |
| Placebo (21) | 44.61±10.12 | · Greater reduction in the fibrosis score and steatosis by TE in synbiotics than placebo (P<0.001) | ||||||
| Sayari et al. [211] (2018) | NAFLD | Taking sitagliptin | Lactobacillus, Bifidobacterium, Streptococcus and fructooligosaccharide | 16 weeks | Synbiotics + sitagliptin (70) | 42.48±11.41 | · Greater reduction of glucose, AST, total cholesterol, and LDL-cholesterol levels in synbiotics than placebo (P<0.05) | RCT |
| Placebo +sitagliptin (68) | 43.42±11.65 | |||||||
| Scorletti et al. [33] (2020) | NAFLD | NAFLD diagnosed by histologic confirmation or imaging evidence of liver fat | Bifidobacterium and fructooligosaccharide | 10–14 months | Synbiotic agents (55) | 50.2±12.4 | · No significant difference in MRS-based liver fat reduction between groups | RCT |
| Placebo (49) | 51.6±13.1 |
Variables are expressed as mean±standard deviation.
NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; AST, aspartate aminotransferase; RCT, randomized controlled trial; ALT, alanine aminotransferase; US, ultrasonography; LDL, low-density lipoprotein; TE, transient elastography; GGT, γ-glutamyl transferase; NAS, NAFLD activity score; LSM, lifestyle modification; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; BMI, body mass index; MRS, magnetic resonance spectroscopy.
Table 7.
| Study | Etiology | Inclusion criteria | Intervention period | Arms (n) | Age (years) | Outcomes | Study design |
|---|---|---|---|---|---|---|---|
| Harrison et al. [233] (2003) | NAFLD | Histologic diagnosis of NASH | 6 months | Vitamin E 1,000 IU/day and C 1,000 mg/day (23) | 50.2 | · Improvement in fibrosis score (47.8% vs. 40.9%; P=0.005) | RCT |
| Placebo (22) | 52.5 | · No changes in inflammation (P>0.05) | |||||
| · ALT improvement (P=0.007) | |||||||
| Sanyal et al. [279] (2004) | NAFLD | Non-diabetic, non-cirrhotic | 6 months | Vitamin E 400 IU/day (10) | 46±13 | · Vitamin E: Improvement in steatosis (P=0.02), ballooning (P=0.055) and portal fibrosis (P>0.05) | RCT |
| Vitamin E 400 IU/day + pioglitazone 30 mg/day (10) | 47±12 | · Vitamin E + pioglitazone: Improvement in steatosis (P=0.002), ballooning (P=0.01), and portal fibrosis (P>0.05) | |||||
| · Comparison between the two groups: steatosis (P<0.05), ballooning (P>0.05), portal fibrosis (P>0.05) | |||||||
| Ersöz et al. [280] (2005) | NAFLD | Histologically proven NAFLD | 6 months | Vitamin E 600 IU/day and C 500 mg/day (28) | 46.3±9.4 | · ALT change (IU/L) (mean, 91.9 to 39.1, P<0.05 vs. 93.7 to 49.1, P<0.05; P>0.05) | Open-label RCT |
| UDCA 10 mg/kg/day (29) | 47.9±10.6 | ||||||
| Dufour et al. [54] (2006) | NAFLD | Histologic diagnosis of NASH | 6 months | UDCA 12–15 mg/kg/day + vitamin E 800 IU/day (15) | 46±14 | · Decrease in AST and ALT levels (IU/L) (UDCA + vitamin E vs. placebo, P<0.05; UDCA vs. placebo, P>0.05) | Double-blinded RCT |
| UDCA 12–15 mg/kg/day + placebo (18) | 47±12 | · Improvement in steatosis (UDCA + vitamin E vs. placebo, P<0.05; UDCA vs. placebo, P>0.05) | |||||
| Placebo + placebo (15) | 44±14 | · No improvement in inflammation and fibrosis (P>0.05) | |||||
| Balmer et al. [55] (2009) | NAFLD | Histologic diagnosis of NAFLD | 2 years | UDCA 12–15 mg/kg/day + vitamin E 800 IU/day (14) | 47±14 | · Adiponectin level change (ng/mL) (mean, +3,808 vs. -1,626 vs. -687; P<0.03) | Double-blinded RCT |
| UDCA 12–15 mg/kg/day + placebo (14) | 47±12 | ||||||
| Placebo + placebo (13) | 46±13 | ||||||
| Sanyal et al. [14] (2010) | NAFLD | Histologic diagnosis of NASH without diabetes | 96 weeks | Pioglitazone 30 mg/day (80) | 47.0±12.6 | · Improvement in NASH (vitamin E 43% vs. placebo 19%, P=0.001; pioglitazone 34% vs. placebo 19%, P=0.04) | Double-blinded RCT |
| Vitamin E 800 IU/day (84) | 46.6±12.1 | · Improvement in fibrosis (vitamin E 41% vs. placebo 31%, P=0.24; pioglitazone 44% vs. placebo 31%, P=0.12) | |||||
| Placebo (83) | 45.4±11.2 | · Changes in serum aminotransferase level (IU/L) (mean, vitamin E -37.0 vs. placebo -20.1, P=0.001; pioglitazone -40.8 vs. placebo -20.1, P<0.001) | |||||
| Aller et al. [232] (2015) | NAFLD | Histologic diagnosis of NAFLD | 3 months | Silymarin 1,080.6 mg/day + vitamin E 72 mg/day (18) | 47.4±11.2 | · Decrease in fatty liver index (mean, 86.2 to 76.9; P<0.05 vs. 85.2 to 77.5; P<0.05) | RCT |
| Control (18) | 43.75±3.5 | · Decrease in NFS (mean, -1.6 to -2.1; P<0.05 vs. -1.0 to -1.5; P<0.05) | |||||
| Parikh et al. [34] (2016) | NAFLD | Non-diabetic, non-cirrhotic | 52 weeks | Vitamin E 800 IU/day (100) | 40.19±2.9 | · ALT normalization (14% vs. 19%; P=0.2) | Open-label RCT |
| UDCA 600 mg/day (150) | · ALT reduction (56% vs. 63%; P=0.2) | ||||||
| Polyzos et al. [229] (2017) | NAFLD | Histologic diagnosis of NASH | 52 weeks | Vitamin E 800 IU/day + spironolactone 25 mg/day (14) | 54.9±1.8 | · NFS reduction (44% vs. 47%; P=0.69) | Open-label RCT |
| Vitamin E 800 IU/day (17) | 53.8±3.4 | · ALT reduction (IU/L) (43.5 to 40.0, P>0.05 vs. 66.0 to 42.1, P>0.05; P>0.05) | |||||
| Bril et al. [237] (2019) | NAFLD | Histologic diagnosis of NASH and type 2 diabetes | 18 months | Vitamin E 800 IU/day (36) | 60±9 | · NAFLD liver fat score reduction (P=0.028 vs. P=0.61) | Double-blinded RCT |
| Vitamin E 800 IU/day + pioglitazone 30-45 mg/day (37) | 60±6 | · Reduction of NAS (vitamin E 31% vs. placebo 19%, P=0.26; vitamin E + pioglitazone 54% vs. placebo 19%, P=0.003) | |||||
| Placebo (32) | 57±11 | · Resolution of NASH (vitamin E 33% vs. placebo 12%, P=0.04; vitamin E + pioglitazone 43% vs. placebo 12%, P=0.005) | |||||
| Fouda et al. [227] (2021) | NAFLD | Histologic diagnosis of NASH | 3 months | Vitamin E 800 IU/day (34) | 44.8±9.7 | · Fibrosis change (vitamin E 50% vs. placebo 30%, P=0.09; vitamin E + pioglitazone 52% vs. placebo 30%, P=0.07) | Single-blind RCT |
| UDCA 500 mg/day (34) | 43.4±11 | · ALT reduction (P<0.05) | |||||
| Pentoxifylline 800 mg/day (34) | 45.2±11 | · Inflammatory cytokine reduction (IL-6, CCL-2/MCP-1) (P<0.05) | |||||
| Kedarisetty et al. [228] (2021) | NAFLD | Histologic diagnosis of NASH | 1 year | Vitamin E 800 IU/day (33) | 35 (16–64) | · ALT reduction (IU/L) (mean 85.5 to 28 vs. 97 to 24; P=0.23) | Open-label RCT |
| Vitamin E 800 IU/day + pentoxifylline 1,200 mg/day (36) | 40 (20–64) | · NAS change (mean 4.3 to 3.1 vs. 5 to 2.8; P=0.45) | |||||
| · Fibrosis stage change (mean 1.7 to 1.7 vs. 2.1 to 1.0; P=0.004) | |||||||
| · Insulin change (mU/L) (mean 12.4 to 10.8 vs. 12.9 to 7.6; P=0.048) | |||||||
| · TNF-α change (pg/mL) (mean 7.14 to 3.83 vs. 7.85 to 1.59; P=0.001) | |||||||
| Groenbaek et al. [248] (2006) | HCV | Elevated ALT | 6 months | Vitamin C 500 mg/day + vitamin E 945 IU/day + selenium 200 µg/day (12) | 45 (33–53) | · Change in serum ALT (IU/L) (mean, -8 vs. -6; P=0.60) | Double-blinded RCT |
| Placebo (11) | 45 (23–55) | · Change in HCV RNA (log10 eqv/L) (mean, 0.17 vs. 0.41; P=0.24) | |||||
| Marotta et al. [246] (2007) | HCV | Cirrhosis, genotype 1, and elevated ALT | 6 months | Vitamin E 900 IU/day (25) | 62 (54–75) | · Improvement of redox status, GSH, GSSG, GSH/GSSG and MDA: vitamin E (P<0.05) and FPP (P<0.05) | RCT |
| Fermented papaya preparation 9 g/day (25) | |||||||
| Control (10) | |||||||
| Bunchorntavakul et al. [249] (2014) | HCV | Genotype 3 | 12 weeks | Vitamin E 800 IU/day (19) | 48.8±8.3 | · Decrease in serum ALT (mean, 105.1 to 96.5; P=0.260 vs. 107.5 to 120.4) | Double- blinded RCT |
| Placebo (18) | 49.5±8.6 | · ALT responder (57.8% vs. 29.4%; P=0.106) | |||||
| Malaguarnera et al. [245] (2015) | HCV | Received PegIFN- α2b + ribavirin | 12 months | Silybin 47 mg/day + vitamin E 15 mg/day + phospholipid 97 mg/day (32) | 46.4±6.9 | · Decrease in ALT level (IU/L) (mean, 170.2 to 36.9, P<0.001 vs. 161.6 to 69.2, P<0.001; P<0.001) | Double- blinded RCT |
| · Decrease in viremia (106 IU/mL) (mean, 5.32 to 1.67, P<0.05 vs. 5.4 to 3.24, P<0.001; P<0.05) | |||||||
| Placebo (32) | 45.2±6.7 | · Decrease in TGF-β (ng/mL) (mean, 54.2 to 32.8, P<0.05 vs. 51.8 to 45.2, P<0.05; P<0.05) | |||||
| · Decrease in PIIINP (ng/mL) (mean, 43.8 to 33.4, P<0.001 vs. 44.7 to 39.8, P<0.05; P<0.05) | |||||||
| · Decrease in TIMP-1 (ng/mL) (mean, 480.2 to 310.6, P<0.001 vs. 487.2 to 421.0, P<0.001; P<0.001) | |||||||
| Andreone et al. [251] (2001) | HBV | Positive HBV DNA and raised ALT (1.5×ULN) | 3 months | Vitamin E 600 IU/day (15) | 37±15 | · ALT normalization (47% vs. 6%; P=0.011) | RCT |
| Control (17) | 42±14 | · Negative HBV DNA (53% and 18%; P=0.039) | |||||
| · Complete response (47% vs. 0%; P=0.0019) |
Variables are expressed as median (interquartile range) or mean±standard deviation.
NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; ALT, alanine aminotransferase; RCT, randomized controlled trial; UDCA, ursodeoxycholic acid; AST, aspartate aminotransferase; NFS, NAFLD fibrosis score; IL-6, interleukin-6; CCL2, C-C motif ligand 2; MCP, monocyte chemoattractant protein; NAS, NAFLD activity score; TNF, tumor necrosis factor; HCV, hepatitis C virus; GSH, glutathione; GSSG, glutathione disulfide, MDA, malondialdehyde; FPP, fermented papaya preparation; PegIFN, pegylated interferon; TGF, transforming growth factor; PIIINP, pro-collagen III N-terminal peptide; TIMP, tissue inhibitor of metalloproteinase; HBV, hepatitis B virus; ULN, upper limit of normal.
Table 8.
| Study | Etiology | Inclusion criteria | Intervention period | Arms (n) | Age (years) | Outcomes | Study design |
|---|---|---|---|---|---|---|---|
| Simon et al. [265] (2018) | Variable | Pooled analysis of cohort | NA | Aspirin (58,855) | 64±8 | · Decreased HCC development in aspirin group (HR, 0.54; 95% CI, 0.36–0.80) | Prospective cohort study |
| Control (74,516) | 62±8 | ||||||
| Petrick et al. [264] (2015) | Variable | Pooled analysis of cohort | NA | Aspirin (477,470) | NA | · Decreased HCC development in aspirin group (HR, 0.68; 95% CI, 0.57–0.81) | Prospective cohort study |
| Control (606,663) | NA | ||||||
| Simon et al. [263] (2019) | NAFLD | Biopsy confirmed NAFLD | NA | Aspirin (151) | 59.9±8.6 | · Decreased prevalence of advanced fibrosis in aspirin group (HR, 0.63; 95% CI, 0.43–0.85) | Prospective cohort study |
| Control (210) | 48.2±13.5 | ||||||
| Shen et al. [262] (2014) | NAFLD | US-confirmed NAFLD | 1 month | NAFLD (2,889) | 54.6±0.3 | · Decreased NAFLD prevalence in aspirin group (HR, 0.62; 95% CI, 0.51–0.74) | Cross-sectional retrospective study |
| Control (8,527) | 48.7±0.5 | ||||||
| Jiang et al. [256] (2016) | CLD | US-confirmed CLD | 1 month | Aspirin (520) | 46.6±15.4 | · Decreased non-invasive fibrosis index in apsirin group, 0.24 standard deviation lower; 95% CI, -0.42 to 0.06 | Cross-sectional retrospective study |
| Control (1,336) | 43.2±14.7 | ||||||
| Hui et al. [271] (2021) | CHB | Receiving nucleos(t)ide analog | Over 90 days | Aspirin (1,744) | 62.2±10.8 | · Decreased HCC development in aspirin group (HR, 0.60; 95% CI, 0.46–0.78) | Retrospective cohort study |
| Control (33,367) | 52.5±12.5 | ||||||
| Choi et al. [267] (2021) | CHB | Over 90 days | Aspirin (7,718) | NA | · Decreased HCC development in aspirin group (OR, 0.92; 95% CI, 0.85–0.99) | Retrospective cohort study | |
| Control (24,977) | NA | ||||||
| Simon et al. [15] (2020) | Viral hepatitis | CHB, CHC monoinfection | Over 90 days | Aspirin (14,205) | 50.5±13.0 | · Decreased HCC development in aspirin group (HR, 0.69; 95% CI, 0.62–0.76) | Prospective cohort study |
| Control (36,070) | 39.6±13.5 | · Decreased liver-related death in aspirin group (HR, 0.73; 95% CI, 0.67–0.81) | |||||
| Liao et al. [270] (2020) | CHC | NA | Aspirin (1,991) | NA | · Decreased HCC development in aspirin group (HR, 0.56; 95% CI, 0.43–0.72) | Retrospective cohort study | |
| Control (1,991) | NA | ||||||
| Lee et al. [268] (2020) | CHC | Over 90 days | Aspirin (2,478) | 63.2±10.0 | · Decreased HCC development in aspirin group (HR, 0.78; 95% CI, 0.64–0.95) | Retrospective cohort study | |
| Control (4,956) | 63.2±10.0 | ||||||
| Lee et al. [269] (2019) | CHB | Over 90 days | Aspirin (2,123) | 58.9±11.8 | · Decreased HCC development in aspirin group (HR, 0.71; 95% CI, 0.58–0.86) | Retrospective cohort study | |
| Control (8,492) | 58.8±11.8 | ||||||
| Lee et al. [276] (2017) | CHB | Low-level viremia | Median 38.5 months | Aspirin (343) | 54.2±11.1 | · Decreased HCC development in aspirin group (HR, 0.26; 95% CI, 0.09–0.74) | Retrospective cohort study |
| Control (1,116) | 50.3±10.8 |
Variables are expressed as median (interquartile range) or mean±standard deviation.
NA, not applicable; HCC, hepatocellular carcinoma; HR, hazard ratio; CI, confidence interval; NAFLD, nonalcoholic fatty liver disease; US, ultrasonography; CLD, chronic liver disease; CHB, chronic hepatitis B; OR, odds ratio; CHC, chronic hepatitis C.
Abbreviations
REFERENCES
- TOOLS
-
METRICS

- ORCID iDs
-
Seung Up Kim

https://orcid.org/0000-0002-9658-8050Yoon Jun Kim

https://orcid.org/0000-0001-9141-7773 - Related articles
-
The effect of moderate alcohol drinking in nonalcoholic fatty liver disease2020 October;26(4)
Beneficial effect of anti-diabetic drugs for nonalcoholic fatty liver disease2020 October;26(4)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



