| Clin Mol Hepatol > Volume 29(4); 2023 > Article |
|
ABSTRACT
ACKNOWLEDGMENTS
FOOTNOTES
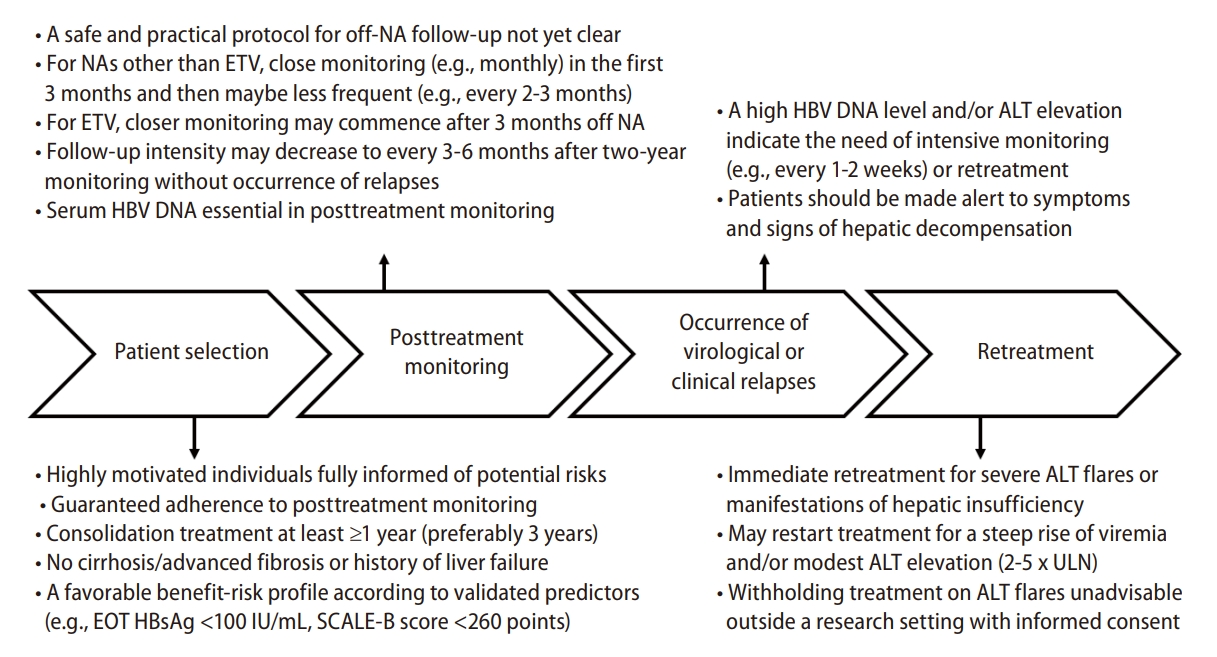
Figure┬Ā1.

Figure┬Ā2.
Table┬Ā1.
Table┬Ā2.
| Guideline | Non-cirrhosis | Cirrhosis |
|---|---|---|
| International guidelines | ||
| AASLD [9] | ┬Ę Indefinite treatment duration (Quality and Certainty of Evidence: Low Strength of Recommendation: Conditional) | ┬Ę Treatment discontinuation not recommended |
| ┬Ę May be considered in patients with HBsAg loss, but evidence insufficient | ||
| APASL [10] | ┬Ę HBsAg loss following either anti-HBsAb seroconversion or at least 12 months of a post-HBsAg clearance consolidation period (B1), or | ┬Ę May be considered with a careful off-therapy monitoring plan (A1) |
| ┬Ę Treatment for Ōēź2 years with consolidation Ōēź1 year (B1) | ||
| EASL [11] | ┬Ę HBsAg loss, with or without anti-HBsAb seroconversion (Evidence level II-2, grade of recommendation 1), or | ┬Ę Treatment discontinuation not recommended |
| ┬Ę May be considered after consolidation Ōēź3 years if close monitoring can be guaranteed (Evidence level II-2, grade of recommendation 2). | ||
| WHO [24] | ┬Ę Life-long therapy in general | ┬Ę Treatment discontinuation not recommended |
| ┬Ę Discontinuation may be considered exceptionally in persons who can be followed carefully long term for reactivation and with persistently normal ALT levels and persistently undetectable HBV DNA levels | ||
| ┬Ę May be considered in persons who have evidence of persistent HBsAg loss and after completion of at least one additional year of treatment, regardless of prior HBeAg status. | ||
| National guidelines | ||
| Canada [25] | ┬Ę HBsAg loss (moderate recommendation; class 2, level B) | ┬Ę HBsAg loss or indefinite duration (moderate recommendation; class 2, level B) |
| China [26] | ┬Ę The therapy aims are ŌĆ£clinical cureŌĆØ (i.e., functional cure) | |
| ┬Ę No recommended criteria for stopping treatment | ||
| ┬Ę No specific recommendations for patients with cirrhosis | ||
| Japan [27] | ┬Ę In general, it is necessary not to stop administration of the NAs | ┬Ę Long-term treatment (Level 5, Grade B) |
| ┬Ę HBsAg loss (can be considered) | ||
| ┬Ę Treatment for Ōēź2 years without detectable HBV DNA or high relapse risk score according to serum HBcrAg and HBsAg levels | ||
| Korea [28] | ┬Ę HBsAg loss (A1). | ┬Ę Long-term treatment (B1) |
| ┬Ę With reference to HBsAg level, cessation of NA therapy could be considered (B1). | ||
| ┬Ę HBcrAg and HBV RNA can be performed when considering cessation of NA therapy (B2) | ||
| Sweden [29] | ┬Ę HBsAg loss (B1) | ┬Ę Long-term treatment (A1) |
| ┬Ę May be considered after long-standing treatment response but require close monitoring after termination(B2) | ||
| Turkey [30] | ┬Ę HBsAg loss | ┬Ę Long-term treatment |
AASLD, American Association for the Study of the Liver; ALT, alanine aminotransferase; APASL, Asian Pacific Association for the Study of the Liver; anti-HBsAb, anti-hepatitis B s-antibody; DNA, deoxyribonucleic acid; EASL, European Association for the Study of the Liver; HBcrAg, hepatitis B core-related antigen; HBeAg, hepatitis B e-antigen; HBsAg, hepatitis B virus s-antigen; HBV, hepatitis B virus; NA, nucleos(t)ide analogue; RNA, ribonucleic acid; WHO, World Health Organization.
Table┬Ā3.
|
Cohort studies |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author (year) | Study type/Region | Number | Age |
Pretreatment status |
Criteria |
Severe adverse events |
||||||
| Cirrhosis | HBeAg(+) | Stopping NA | Resuming NA | Severe hepatitis flares or decompensation┬¦ | Death or liver transplantation | Risk factors | ||||||
| RETRACT-B Hirode et al. [33] (2023) | Prospective/International | 1,557 | 52.9ŌĆĀ | 11.8% | 15.8% | As pre institution | As per institution | 20 (1.3%) | 4 (0.3%)Ōłź | ┬Ę iCirrhosis | ||
| Year | Incidence | 95%CI | ┬Ę iPretreatment HBeAg(+) | |||||||||
| 1 | 1.0% | 0.6ŌĆō1.6% | ||||||||||
| 2 | 1.4% | 0.9ŌĆō2.2% | ||||||||||
| 3 | 1.6% | 1.1ŌĆō2.5% | ||||||||||
| 4 | 1.8% | 1.1ŌĆō3.0% | ||||||||||
| 5 | 1.8% | 1.1ŌĆō2.0% | ||||||||||
| Hsu et al. [34] (2022)* | EHR/Taiwan | 665 | 50.3ŌĆĪ | 14.3% | 26.0% | Taiwan reimbursement | Taiwan reimbursement | 24 (3.6%) | 2 (0.3%) | ┬Ę iCirrhosis | ||
| i┬Ę Male | ||||||||||||
| Liu et al. [35] (2022) | Prospective/Taiwan | 1,234 | 56.4ŌĆĀ | 40.1% | 0% | APASL 2012 | Taiwan reimbursement | Hepatitis flares: 516 (41.8%) | 5 (0.4%) | ┬Ę iCirrhosis ┬Ę iPrior-treatment | ||
| Decompensation: 13 (1.1%) | ┬Ę iAge (cut-off: 55) ┬Ę iGenotype B | |||||||||||
| ┬Ę iTDF (vs. ETV) | ||||||||||||
| ┬Ę iPretreatment viral load (cut-off: 6 log IU/mL) | ||||||||||||
| ┬Ę iPretreatment-HBsAg (cut-off: 3 log IU/mL) | ||||||||||||
| ┬Ę iEOT-HBsAg (Ōēź500 vs. <100 log IU/mL) | ||||||||||||
| PS: the predictors for hepatitis flares, not for decompensation | ||||||||||||
| Hsu et al. [41] (2021) | EHR/Taiwan | 10,192 | 50.9ŌĆĀ | 10.7% | n.a. | Taiwan reimbursement | Taiwan reimbursement | 132 (1.3%) | 51 (0.5%) | ┬Ę iCirrhosis | ||
| 4 year: 1.8% (95% CI, 1.5ŌĆō2.2%) | 4 years: 0.7% (95% CI, 0.5ŌĆō1.9%) | ┬Ę iMale | ||||||||||
| ┬Ę iAge (cutoff age, 50 years) | ||||||||||||
| ┬Ę iHistory of liver failure | ||||||||||||
| Ma et al. [44] (2019) | Prospective and retrospective/Taiwan | 535 | 50.7ŌĆĀ | 0% | 29.9% | APASL 2012 | Taiwan reimbursement | 7 (1.3%) | 1 (0.2%) | n.a. | ||
| CREATE Sonneveld et al. [45] (2022) | Prospective and retrospective/International | 572 | 52.0ŌĆĪ | n.a. | 16.6% | As per institution | As per institution | 2 (0.4%) | 0 (0%) | n.a. | ||
| Wong et al. [46] (2020) | EHR/Hong Kong | 1,076 | 59.1ŌĆĀ | 8.3% | 0% | n.a. | n.a. | 7 (0.7%) | n.a. | n.a. | ||
|
Meta-analysis study |
||||||||||||
| Author (year) | Enrolled studies | Main finding | ||||||||||
| Tseng et al. [43] (2022) | 50 articles reporting safety outcomes after NA cessation | ┬Ę Heterogeneous design among studies (e.g., stopping rules, retreatment criteria, and definition of decompensation) | ||||||||||
| 15 studies (4,525 patients) pooled for risk estimate of overall population | ┬Ę Serious adverse events not often reported in smaller studies with shorter follow up duration | |||||||||||
| 14 studies (3,731 patients) pooled for risk estimate of non-cirrhotic population | ┬Ę Risk estimate (95% CI): | |||||||||||
| 5 studies (744 patients) pooled for risk estimate of cirrhotic population | Overall | Non-cirrhosis | Cirrhosis | |||||||||
| Severe | 1.2% | 0.9% | 3.6% | |||||||||
| flares/Decompensation | (0.7ŌĆō2.1%) | (0.4ŌĆō1.8%) | (1.8ŌĆō7.3%) | |||||||||
| Death/Liver | 0.4% | 0.3% | 1.0% | |||||||||
| transplantation | (0.2ŌĆō0.7%) | (0.1ŌĆō0.7%) | (0.5ŌĆō2.1%) | |||||||||
For studies from similar institutions, we chose the most representative one such as the larger sample size or more detailed information about the adverse events.
APASL, Asian Pacific Association for the Study of the Liver; CI, confidence interval; EOT, end-of-treatment; EHR, electronic health record; ETV, entecavir; HBeAg, hepatitis B e-antigen; HBsAg, hepatitis B s-antigen; n.a., not available; NA, nucleo(s)tide analogue; TDF, tenofovir disoproxil fumarate.
Ōłź the number from another publication from RETRACT-B cohort. [48]
Table┬Ā4.
| Author (year) | Race/Region/Setting | Number | Age (year) | Male (%) | Pre-treatment HBeAg (+) | ETV/TDF proportion | Cirrhosis | Stopping rule | Duration (month): Treatment/Consolidation/Follow-up | Event: Clinical relapse/HBsAg loss |
Performance of the SCALE-B |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical relapse | HBsAg loss | |||||||||||
| Hsu et al. [62] (2019) | Asian/Taiwan/Prospective Multicenter | 135 | 49.5* | 80.7 | 31 (22.9%) | 100% | 0% | Treatment duration Ōēź3 years with undetectable HBV DNA and negative HBeAg on treatment cessation | 36.7*/25.2*/25.9* | 66 (48.9%)Ōłź/8 (5.9%) | AUC**: | 3-year incidence** |
| 1Y: 0.87 (0.80ŌĆō0.93) | High risk: 0 | |||||||||||
| 3Y: 0.87 (0.79ŌĆō0.94) | Intermediate: 0 | |||||||||||
| 5Y: 0.90 (0.79ŌĆō1.00) | Low risk: 27.1% (14.5ŌĆō47.3%) | |||||||||||
| 5-year cumulative | ||||||||||||
| incidence** | ||||||||||||
| High risk: 86.2% (67.8ŌĆō96.8%) | ||||||||||||
| Intermediate: 61.6% (48.2ŌĆō75.2%) | ||||||||||||
| Low risk: 17.2% (7.5ŌĆō36.9%) | ||||||||||||
| CREATE Sonneveld et al. [45] (2022) | Mixed (Asian:79.9%)/ International/Prospective and retrospective | 572 | 52* | 68.2 | 16.6% | 77.8% | n.a. | Per institution | 73.8*/n.a./12┬¦ | 92 (16.1%)ŌĆĪ/24 (4.2%) | Proportion at week 48┬¦ | Proportion at week 48┬¦ |
| High risk: 31% | High risk: 1% | |||||||||||
| Intermediate: 14% | Intermediat: 2% | |||||||||||
| Low risk: 3% | Low risk: 11% | |||||||||||
| Liao et al. [65] (2021) | Asian/China/Prospective Single center | 122 | 34* | 77.9 | 100% | 58.2% | 0% | APASL 2012 | 56.4*/30.0*/36.0* | 44 (36.1%)Ōłź/12 (9.8%) | AUC** | Not mentioned |
| 1Y: 0.81 (0.73ŌĆō0.89) | ||||||||||||
| 3Y: 0.74 (0.65ŌĆō0.84) | ||||||||||||
| 5Y: 0.75 (0.65ŌĆō0.85) | ||||||||||||
| 5-year cumulative | ||||||||||||
| incidence** | ||||||||||||
| High risk: 82.2% | ||||||||||||
| Intermediat: 50.0% | ||||||||||||
| Low risk:22.2% | ||||||||||||
| Papatheodoridis et al. [66] (2020) | Caucasian/Greece/Prospective Multicenter | 57 | 60*,ŌĆĀ | 64.9 | 0% | 100% | 0% | Treatment duration Ōēź4 years, ETV or TDF Ōēź2 years, and undetectable HBV DNA Ōēź3 years | n.a./63.6*/19.0* | 19 (33.3%)Ōłź/12 (21.1%) | No association | Not mentioned |
| Kaewdech et al. [91] (2022) | Asian/Thailand/Prospective Single center | 92 | 55.0* | 64.1 | 21.7% | 44.6% | 0% | APASL 2016 | 78.0*/n.a./35.5 | 31 (33.7%)/7 (7.6%) | AUC: | Proportion at week 96 |
| 2Y: 0.81 | High risk: 0% | |||||||||||
| Intermediat: 2.4% | ||||||||||||
| Low risk: 14.3% | ||||||||||||
All the status are at the cessation of antiviral therapy unless otherwise specified.
ALT, alanine aminotransferase; APASL, Asian Pacific Association for the Study of the Liver; AUC, area under ROC curve; CI, confidence internal; DNA, deoxyribonucleic acid; EOT, end of treatment; ETV, entecavir; HBcrAg, hepatitis B core-related antigen; HBeAg, hepatitis B e-antigen; HBsAg, hepatitis B s-antigen; n.a., not available; NA, nucleos(t)ide analogue; RNA, ribonucleic acid; TDF, Tenofovir disoproxil fumarate.
Table┬Ā5.
| Institution/Study, Site | Retreatment criteria |
|---|---|
| Criteria used in randomized controlled trials | |
| FINITE study, [102] Multicenter in Germany | At least one of the criteria: |
| ┬Ę Increase of direct bilirubin by >1.5 mg/dL (>25 ╬╝mol/L) from baseline, and ALT >ULN | |
| ┬Ę Increase in PT Ōēź2.0 s (INR Ōēź0.5) prolonged from baseline with adequate vitamin K therapy, and ALT >ULN | |
| ┬Ę ALT >10X ULN with or without associated symptoms. | |
| ┬Ę ALT >2X ULN and Ōēż5X ULN persisting for Ōēź84 days (12 weeks), and HBV DNA >20,000 copies/mL (equivalent to 357 IU/mL) | |
| ┬Ę ALT >5X ULN and Ōēż10X ULN persisting for Ōēź28 days (4 weeks). | |
| Stop-NUC study, [103] Multicenter in Germany | At least one of the criteria: |
| ┬Ę ALT >10X ULN | |
| ┬Ę 10X ULN ŌēźALT>5X ULN for Ōēź28 days | |
| ┬Ę 5X ULN ŌēźALT>2X ULN for Ōēź112 days and HBV DNA >2,000 IU/mL | |
| Increase of total bilirubin by >1.5X ULN | |
| Toronto-STOP study, [104] Toronto Centre for Liver Disease, Canada | At least one of the criteria: |
| ┬Ę HBeAg seroreversion | |
| ┬Ę HBV DNA >2,000 IU/mL and ALT >600 IU/mL at any visit | |
| ┬Ę HBV DNA >2,000 IU/mL and ALT >200 IU/mL (5X ULN) on two consecutive visits | |
| ┬Ę HBV DNA >2,000 IU/mL and ALT >200 IU/mL but <600 IU/mL for >6ŌĆō8 weeks | |
| ┬Ę HBV DNA >20,000 IU/mL on two consecutive visits at least 4 weeks apart. | |
| Criteria used in prospective observational study | |
| Queen Mary Hospital64, Hong Kong | ┬Ę Virological relapse: HBV DNA >2,000 IU/mL |
| Australia multicenter study, [105] Australia | At least one of the criteria: |
| ┬Ę HBV DNA >2,000 IU/mL and serum ALT >5X ULN for Ōēź16 weeks or ALT >10X ULN for Ōēź8 weeks | |
| ┬Ę Clinical evidence of hepatic decompensation defined by INR Ōēź1.5 or bilirubin >2X ULN or ascites or hepatic encephalopathy Investigator discretion | |
| DARING-B study, [106] Laiko General Hospital and Hippokration General Hospital, Greece | At least one of the criteria: |
| ┬Ę ALT >10X ULN | |
| ┬Ę ALT >5X ULN and total bilirubin >2 mg/dL at the same visit | |
| ┬Ę ALT >3X ULN and HBV DNA >100,000 IU/mL at the same visit | |
| ┬Ę ALT >ULN and HBV DNA >2,000 IU/mL on three sequential visits. | |
| ┬Ę According to patientsŌĆÖ and physiciansŌĆÖ decisions in case of virological relapse with HBV DNA >20,000 IU/mL | |
| Nanfang Hospital, [65,107] China | Clinical relapse: HBV DNA >2,000 IU/mL and ALT >2X ULN |
| Multiple centers in China [108] | |
| Taiwan National Health Insurance, [71] Taiwan | ALT >2X ULN with 3 months apart and HBV DNA >2,000 IU/mL or total bilirubin >2 mg/dL, or prolongation of PT Ōēź3 seconds |
Table┬Ā6.
| Study | Scale/Region | Primary outcome | Key inclusion criteria | Group | Age/Caucasian/Male/Fibroscan (kPa) | ETV or TDF (%)/Pre-Tx HBeAg (+)/HBsAg (log IU/mL)/NA duration (months) | Clinical relapse, number (%) | HBsAg loss, number (%) | Adverse events, number (%) |
|---|---|---|---|---|---|---|---|---|---|
| FINITE, Berg et al. [102] (2017) | Multicenter/Germany | HBsAg loss or seroconversion at week 144 | TDF Ōēź4 years | Stop: n=21 | 44.5ŌĆĀ/18 (85.7%)/18 (85.7%)/6.1ŌĆĀ | 21 (100%)/0 (0%)/4.4ŌĆĀ/n.a. | At least 5 (23.8%)**,ŌĆĀŌĆĀ | 4 (19.0%) | Grade 3/4: 5 (23.8%) |
| HBV DNA <400 copies/mL Ōēź3.5 years | |||||||||
| Pre-Tx HBeAg(-) | |||||||||
| No advanced fibrosis/cirrhosis (by histology or Fibroscan) | Continue: n=21 | 45.5ŌĆĀ/19 (90.5%)/15 (71.4%)/5.0ŌĆĀ | 21 (100%)/0 (0%)/4.6ŌĆĀ/n.a. | 1 (4.8%)**,ŌĆĀŌĆĀ | 0 (0%) | Grade 3/4: 0 (0%) | |||
| No history of decompensation | |||||||||
| Stop-NUC*, van B├Čmmel et al. [103] (2020) | Multicenter/Germany | HBsAg loss at week 96 | NA Ōēź4 years | Stop: n=79 | 51.6ŌĆĀ/62 (78.5%)/50 (63.3%)/5.7ŌĆĀ | 71 (89.9%)/0 (0%)/3.5ŌĆĀ/n.a. | 28 (35.4%)ŌĆĪ | 10 (12.7%) | n.a. |
| HBV DNA <1,000 IU/mL Ōēź4 years | |||||||||
| Pre-Tx HBeAg(-) | |||||||||
| Pre-Tx HBV DNA >2,000 IU/mL | |||||||||
| No advanced fibrosis/cirrhosis (by histology of Fibroscan) | Continue: n=79 | 52.0ŌĆĀ/68 (82.2%)/51 (64.6%)/5.7ŌĆĀ | 72 (91.1%)/0 (0%)/3.6ŌĆĀ/n.a. | 0 (0%)ŌĆĪ | 0 (0%) | n.a. | |||
| Toronto-STOP, Liem et al. [104] (2019) | Single center/Canada | HBV DNA <2,000 IU/mL at week 48 | NA Ōēź1 year | Stop: n=45 | 59ŌĆĀ/2%/26 (57.8%)/4.9ŌĆĀ | 45(100%)/27 (60.0%)/3.1ŌĆĀ/72.0ŌĆĀ | At least 10 (22.2%)┬¦,Ōłź | 1 (0.2%)Ōłź | 22(48.9%): ALT >5X ULN |
| Consolidation: | 1 (2.2%): bilirubin >66 ╬╝mol/L | ||||||||
| Pre-Tx:HBeAg(+): 1 year and HBeAb(+) | |||||||||
| Pre-Tx HBeAg(-): 3 years | Continue: n=22 | 50ŌĆĀ/5%/14 (63.6%)/5.2ŌĆĀ | 22 (100%)/13 (59.1%)/3.0ŌĆĀ/61.2ŌĆĀ | 0 (0.0%)┬¦,Ōłź | 1 (0.5%)Ōłź | 0 (0.0%) | |||
| No cirrhosis (defined by histology or Fibroscan) |
Abbreviations
REFERENCES
-
METRICS

- ORCID iDs
-
Jia-Horng Kao

https://orcid.org/0000-0002-2442-7952 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



