| Clin Mol Hepatol > Volume 27(1); 2021 > Article |
|
ABSTRACT
Autoimmune hepatitis (AIH) is an immunoinflammatory chronic liver disease with dynamic and rather heterogeneous disease manifestations. A trend of increasing prevalence of AIH has been observed worldwide, along with a relative increase in the percentage of male patients. AIH is characterized and diagnosed based on serum biochemistry and liver histology: elevated aminotransferases and serum immunoglobulin G (IgG), the presence of serum anti-nuclear antibody or anti-smooth muscle antibody, and interface lympho-plasmacytic hepatitis. Clinical manifestations differ among disease subtypes with distinct time-frames, i.e., AIH with a chronic insidious onset, and acute-onset AIH (the diagnosis of which is often challenging due to the lack of typical serum findings). The absence of disease-specific biomarkers or histological findings may expand the disease phenotype into drug-induced AIH-like liver injury. Corticosteroids and azathioprine are recommended first-line treatments for AIH. The complete normalization of aminotransferases and serum IgG is an essential treatment response to ensure long-term overall survival. An incomplete response or intolerance to these drugs is considered an indication for second-line treatment, especially with mycophenolate mofetil. Life-long maintenance treatment is required for the majority of patients, but the few who achieve prolonged and stringent biochemical remission with lower alanine aminotransferase and IgG within the normal range may be able to discontinue the medications. In the future, the quality of life of AIH patients should be managed by personalized medicine, including the appropriate selection and dosing of first-line therapy and perhaps alternating with potential therapeutics, and the prediction of the success of treatment withdrawal.
Autoimmune hepatitis (AIH) is an immune-mediated inflammatory liver disease of non-self-limiting clinical course for which immunosuppressive agents are necessary in the majority of affected patients. The concept of the immunopathogenesis of AIH relies on autoreactive CD4 and CD8 T cells, whose emergence is induced after the break of self-tolerance by environmental triggers [1]. As the inflammation that AIH presents is likely to be characterized by a dynamic transition of the milieu of multiple effector immune cells in the liver, clinicians should take into consideration the chronological dynamics of disease manifestations or of distinct subtypes of disease, e.g., ranging from acute-onset, acute on chronic, and chronic insidious manifestation. The appropriate diagnosis and proper treatment strategy with special attention to the clinical subtypes of AIH must be considered to ensure favorable short- and long-term survival.
In 2019, the American Association of the Study of Liver Disease (AASLD) published very comprehensive practice guidance and guidelines for AIH that updated the previous version published in 2010 [1]. The progress in our understanding of AIH is apparent in these guidelines, including their detailed description of a first-line treatment strategy based on the patient’s clinical manifestations. In this review, we summarize the recent updates regarding the management of AIH, focusing on the disease manifestations (Fig. 1) and the time frame of treatment and responses to treatment.
AIH affects individuals of all ages from children to the very elderly, but it is most commonly identified in middle-aged women [2,3] in all ethnic groups. In 2016, a nationwide, hospital-based, epidemiological survey to approximate the prevalence of AIH was carried out in Japan. The estimated number of patients was 30,330 (95% confidence interval [CI], 29,592–31,069) and the calculated point prevalence of AIH per 100,000 population was 23.9 (95% CI, 23.3–24.5) [4]. Compared to the previous survey in 2004, the data revealed an almost threefold increase in the prevalence of AIH [4].
Among the widely varying nation-based prevalence data for adult AIH reported after 2000, e.g., from 4.0 (Singapore) [5] to 42.9 (Alaska) [6], a trend of increasing prevalence has been observed worldwide; for instance, from 10.7 in 2003 [7] to 17.3 in 2009 [8] in Sweden. The prevalence of AIH in Korea increased gradually from 2009 to 2013, although the incidence remained stable [2]. Alterations of environmental factors, including changes in lifestyle, might trigger the development of AIH, and environmental factors are likely to be linked to the increased male to female ratio of AIH in Japan from 1:6.9 in 2004 to 1:4.3 in 2016 as shown by the aforementioned survey [4]. Improved awareness of AIH among clinicians worldwide might also have contributed to the trend of increased prevalence, possibly resulting in a reduction of the number of otherwise undiagnosed patients, including adult male patients.
AIH is a disease without signature diagnostic features. The diagnosis of AIH requires 1) histological abnormalities (interface hepatitis), 2) characteristic laboratory findings (elevated serum hepatic enzymes, aspartate aminotransferase [AST] and alanine aminotransferase [ALT], and increased serum immunoglobulin G [IgG]), and 3) positive results of disease-defining autoantibodies, coupled with 4) the exclusion of other liver diseases that may resemble AIH, including viral hepatitis, hereditary, metabolic, cholestatic, or drug-induced liver injury (DILI). Anti-nuclear antibodies (ANA) and anti-smooth muscle antibodies should be tested in patients of all ages, and an additional test of anti-liver kidney microsomal type 1 is necessary in children for the characterization of type 2 AIH [1]. The clinical judgement is straightforward in typical AIH patients with the above-mentioned hallmarks, but atypical cases should be diagnosed with the aid of diagnostic scoring systems that were originally developed by the International AIH Group (IAIHG) in 1993 for the identification of patients with AIH for clinical research [9]. The revised IAIHG criteria reported in 1999 [10] and the simplified criteria proposed in 2008 [11] are commonly implemented in clinical practice, and they emphasize distinct diagnostic values.
As the simplified scoring has superior specificity (90% vs. 73%) and accuracy (92% vs. 82%) compared to the revised scoring system [12], the former is preferable for the diagnosis of typical AIH cases. On the other hand, the revised scoring system is suitable for the reassessment of atypical cases with a low score in the simplified system, including cases of autoantibody-negative hepatitis and acute-onset AIH with normal IgG values [1]. Limitations to both scoring systems are evident (due to the lack of accuracy) for a diagnosis of AIH that is overlapped with a primary biliary cholangitis (PBC) [13], primary sclerosing cholangitis (PSC), or non-alcoholic fatty liver disease (NAFLD) [14], or fulminant liver failure.
The diagnosis of AIH requires liver biopsy results presenting compatible histological abnormalities. Typical histological features are indicative of (chronic) active hepatitis, comprising lymphoplasmacytic interface hepatitis, emperipolesis (intrusion of one intact lymphocyte into a hepatocyte), and hepatocyte rosettes. Gurung et al. [15] recently hypothesized that typical histological features are related to the severity of disease, but not to the etiology itself, and they reported the following as AIH-specific histological features: 1) Kupffer cell hyaline granules, 2) prominence of plasma cells in portal tracts, and 3) the relative predominance of plasma cells over lymphocytic inflammation. After Gurung et al. [15] adjusted the analysis results for the inflammatory grade, emperipolesis and rosette formation were similarly found in the disease control, chronic hepatitis C (CHC). The Kupffer cell hyaline granules were well-circumscribed, eosinophilic periodic acid-Schiff diastase-resistant deposits within Kupffer cells, and they were originally proposed as a specific histology in pediatric AIH [16].
Centrilobular necrosis is another histological AIH feature, presenting in a rather disease manifestation-specific manner in acute-onset AIH [17] and in acute liver failure (ALF). In ALF, central perivenulitis, plasma cell-enriched inflammatory infiltrate, and lymphoid follicles on a background of massive hepatic necrosis are the principle findings [18].
In clinical practice, the differential diagnosis of AIH in liver histology is routinely focused on DILI, including drug-induced AIH (DIAIH)-like liver injury. Though the rare presence of bridging fibrosis and the absence of advanced fibrosis are clues suggesting DILI, this is not the case for the differential diagnosis of acute-onset AIH over DILI.
Histological findings of NAFLD and non-alcoholic steatohepatitis (NASH) are reported to be present in 17–30% of adult AIH patients [19,20]. These overlapping findings are indicative of patients who are at increased risk of liver-related mortality [19]. Conversely, characteristic laboratory findings with positive autoantibodies (especially in female patients) are sometimes refuted by the mere histology of NAFLD or NASH in the liver. Signature diagnostics for discriminating NASH with prominent periportal hepatitis from chronic active AIH are greatly anticipated.
The long-term outcome of AIH is associated with the stage of fibrosis. Since the evaluation of liver fibrosis by biopsy during the course of disease management is not feasible, noninvasive assessments have been conducted in clinical hepatology by using serum biomarkers, including the serum AST/platelet ratio index (APRI) and the fibrosis-4 (FIB-4) index [21]. However, a recent systemic review of the diagnostic accuracy of APRI and FIB-4 demonstrated their poor performance for detecting advanced fibrosis and cirrhosis in AIH [22].
Noninvasive assessment by liver stiffness has been shown to identify advanced fibrosis and cirrhosis in AIH with reasonable accuracy. The performance levels of vibration-controlled transient elastography (VCTE) and magnetic resonance elastography were indicated to be superior to those of the APRI and FIB-4, and VCTE was validated in a systemic review as providing good performance [22]. Considering that liver inflammation affects liver stiffness, the stiffness value at the initial diagnosis before the initiation of treatment with immunosuppressive agents is confounded by disease activity. In fact, the value of VCTE within 3 months after the start of treatment was significantly correlated with histological grading, but not with the fibrosis stage [23]. Thereafter, at least 6 months after the successful treatment of AIH, the area under the receiver operating curve of VCTE reached 1.0 [23]. Sustaining biochemical remission (normal ALT and normal IgG) and the use of VCTE help monitor and manage the disease course of AIH.
A novel serum fibrosis marker, i.e., Mac-2 binding protein glycosylation isomer (M2BPGi), which was originally reported to be associated with the fibrosis stage in CHC patients [24], is likely to become an alternative to the use of VCTE; the M2BPGi value is influenced by both inflammation and fibrosis in AIH patients, in a similar way to VCTE [25]. A ‘one-serum parameter fits all’ approach to the evaluations of disease activity and fibrosis could be achievable with serum M2BPGi, but further studies are necessary to validate its utility.
Acute-onset AIH is a clinically challenging disease subtype because a delayed diagnosis and delayed treatment, especially in the absence of typical serological findings, may lead to a poorer short-term prognosis. The prevalence of acute-onset AIH has been obtained in several cross-sectional studies worldwide. The 2019 AASLD practice guidance and guidelines state that 25–75% of individuals with AIH in western countries present with an acute onset and a disease duration <30 days [1,26]. A Korean study reported the prevalence 46.4%, using almost the same definition [27]. An Italian multicenter cohort applied arbitrary criteria, i.e., >10× the upper limit of normal (ULN) of transaminases and >5 mg/mL of bilirubin, and the study’s authors reported that 43% of their series of AIH patients were acute-onset [28]; among the patients who underwent liver biopsy, 64.8% fulfilled the histological criteria for acute-onset AIH, with the fibrosis stage lower than Ishak F2. In a Japanese nationwide cross-sectional study of AIH patients diagnosed in 2009–2013, the frequency of acute hepatitis without fibrosis (F0) was, on the other hand, almost 11% [3].
Acute-onset AIH may encompass two distinct clinical subgroups: 1) ‘genuine’ acute AIH with no chronic liver pathology (portal, followed by bridging fibrosis) and 2) acute exacerbation of chronic AIH. Even among the group of genuine acute AIH cases, dynamic histological changes—especially in the extent of portal fibrosis—are anticipated. As a trend, the median duration between disease onset and liver biopsy among Japanese AIH patients with acute presentation was longer in the F1–2 patients than in the F0 patients (29 vs. 15 days, P=0.052) [29]. Nevertheless, there were no significant differences between these two groups in laboratory data, AIH-related pathological findings, or disease outcomes after the introduction of prednisolone.
Typical serological hallmarks of AIH (e.g., positive autoantibody or elevated serum IgG) are frequently absent in acute-onset AIH; in the above-mentioned Japanese cohort, 27% of the patients were ANA-negative (<×40) and >50% of them had normal serum IgG values [29]. As the absence of ANA and normal serum IgG were not associated with disease outcomes in that cohort, the prompt initiation of treatment with immunosuppressive agents was necessary to prevent progression to acute severe AIH (AS-AIH), and eventually ALF.
AS-AIH is defined by the AASLD as AIH with jaundice, a prothrombin time (PT) international normalized ratio (INR) ≥1.5, and neither encephalopathy nor previously recognized liver disease (Table 1) [1]. A thorough diagnosis protocol that includes a transjugular liver biopsy is needed to differentiate AS-AIH from acute severe hepatitis with multiple other etiologies. In a cohort from the UK and France, 69% [30] and 59% [31] of the AS-AIH cases were reported to progress to ALF-AIH, respectively. A continuum of treatment strategies that are based on the benefit-to-risk ratio of glucocorticoid therapy should thus be seriously considered (Fig. 2). In the 2019 AASLD practice guidance, prednisone or prednisolone monotherapy (60 mg/day in adults) is recommended for AS-AIH [1], because no association with an increase in sepsis was demonstrated [32].
A short-term treatment response (within 1–2 weeks) in AS-AIH is indeed crucial to prevent disease progression. Zachou et al. [33] recently observed that high-dose intravenous (iv.) corticosteroids (either 1 g methylprednisolone for 3 consecutive days followed by iv. 1 mg/kg/day prednisolone, or iv. 1.5 mg/kg/day prednisolone) was safe and effective to treat AS-AIH patients (n=34; all were F0–2, and transaminases were >10× ULN). The complete response rate was higher than that in the non-AS-AIH group, with no case requiring liver transplantation (LT).
AS-AIH with encephalopathy is defined as ALF caused by AIH (ALF-AIH) (Table 1). With regard to noninvasive diagnoses, heterogenous hypo-attenuated regions within the liver as visualized by unenhanced computed tomography (CT) is useful to differentiate patients with AS/ALF-AIH from those with viral-associated ALF [34]. The volumetric measurement of the liver on CT is also valuable, because the size of the liver was reported to be significantly reduced in non-acetaminophen cases of acute liver injury/ALF compared to the acetaminophen-induced cases [35]. A direct evaluation of indications for LT is recommended in ALF-AIH, because glucocorticoid therapy has not been associated with improved overall survival and is even harmful to patients with a model for end-stage liver disease (MELD) score >40 [36].
ACLF is caused by an AIH flare in previously diagnosed or undiagnosed chronic liver disease/cirrhosis (AIH-ACLF) patients. The Asian Pacific Association for the Study of Liver ACLF Consortium defines ACLF as patients with jaundice (bilirubin >5 mg/dL) and coagulopathy (PT [INR] ≥1.5), complicated by ascites and/or encephalopathy within 4 weeks after diagnosis. The consortium reported that 2.9% (n=82) of the ACLF cases diagnosed in 2012–2017 in nine Asian countries were regarded as having developed AIH as an acute insult; 97% of the patients exhibited IgG elevation (>1.1× ULN), whereas 49% were seronegative for autoantibodies [37]. Although 34% of the patients (n=28) being treated with a corticosteroid showed a significantly improved 90-day survival rate compared to those without treatment (75% vs. 48.1%, P=0.02), early stratification to corticosteroid therapy or LT is necessary [37]; predictors of an unfavorable response to corticosteroids were revealed to include a MELD score >27 and hepatic encephalopathy in advanced fibrosis (≥F3).
DILI can occasionally be diagnosed based on increased serum IgG and positive ANA. Even after the cessation of suspected drugs, such ‘AIH-mimic’ patients whose ALT elevation is persistent or progressive are indicated for treatment with immunosuppressive agents to prevent ALF. Remarkably, the short-term (1 week) response to corticosteroids was demonstrated to be more pronounced in the patients with AIH-mimic DILI compared to those with pure AIH [38].
AIH-mimic DILI and pathogenically DIAIH are difficult to differentiate by liver pathology, including the intensity of inflammatory infiltrates, the type of the predominant inflammatory cells, and the grade of fibrosis. In the 2019 AASLD practice guidance and guidelines, the term “DIAIH-like injury” was introduced as an alternative to DIAIH [1]. The majority of patients with DIAIH-like injury are acute-onset, and up to 30% of the cases are accompanied by hypersensitivity reaction [39]; the latency periods of minocycline and nitrofurantoin (the two most commonly implicated drugs) can exceed 12 months [40]. HLA-DR3 or -DR4 and cirrhosis at presentation are unusual [41]. Fulfilling Hy’s law, serum aminotransferase levels >3× ULN and total serum bilirubin >2× ULN as a predictor of ALF in patients with DILI [42], or failures of improvement in laboratory tests after medication discontinuation are the reasons for the implementation of glucocorticoids.
The outcome of DIAIH-like injury has been shown to be excellent, and relapse after glucocorticoid withdrawal is rare [43]. Emerging liver injury that is related to the use of immune checkpoint inhibitors is distinct from DIAIH-like injury, as it lacks the typical serological and histological features of AIH [44,45].
The concurrences of AIH and PBC or AIH and PSC are not confirmed as specific pathological entities, but the identification of clinical ‘overlap’ among AIH patients is of importance, as these patients may not obtain sufficient benefit with only conventional AIH treatment.
Concerning the AIH-PBC overlap, it should be noted that 5–35% of AIH patients, even in the absence of bile duct lesions, are reported to be positive for the serological hallmark of PBC, i.e., anti-mitochondrial antibodies (AMAs) [46]. Simultaneous and sequential AIH-PBC overlaps should be considered separately; the former is suspected in the presence of destructive cholangitis at the initial diagnosis, and the latter is suspected based on the occasional elevation of cholestatic enzymes after biochemical remission. The ‘Paris criteria’ for the identification of AIH-PBC overlap is valuable for apparent cases [47], but this criteria may miss cases with less severe cholestatic features [48,49]. The IAIHG’s position statement did not endorse the Paris criteria or even the revised AIH criteria regarding the diagnosis of AIH-PBC overlap [13]. Nevertheless, the reevaluation of suspected AIH-PBC overlap patients in light of their responses to immunosuppressive agents is likely practical and necessary.
AIH-PSC overlap is diagnosed based on the following: 1) the typical features of AIH, 2) the absence of AMA, and 3) evidence of large-duct PSC by endoscopy or magnetic resonance imaging or of small-duct PSC, confirmed by ‘onion-skinning’ periductal fibrosis in a liver biopsy [49]. Concurrent ulcerative colitis with AIH is a critical indication for AIH-PSC overlap, especially in pediatric patients.
The cases of hepatitis C virus-infected patients are occasionally accompanied by positive serum and histological markers of AIH, making a differential diagnosis of CHC-AIH overlap syndrome necessary. Direct-acting antiviral (DAA) therapy for CHC patients with AIH features was shown to significantly decrease ALT into the normal range, and serum markers of AIH in those patients began decreasing by 6 months post-treatment; >50% of the patients achieved complete resolution [50]. The CHC-AIH overlap syndrome may be a historical disease entity that is not likely to be diagnosed after the era of DAA.
The purposes of the treatment of AIH are to first relieve symptoms, and then to achieve a biochemical response, control hepatic inflammation toward histological remission, prevent disease progression, and promote the regression of fibrosis. The ideal biochemical response, regarded as biochemical remission by the AASLD, is the normalization of the patient’s serum AST, ALT, and IgG levels to within the ULN (Table 1) [1]. A favorable treatment response in AIH patients assures overall survival that is comparable to that of general populations [51]. Due to the heterogenous manifestations of AIH, short- and long-term treatment responses with regard to liver-related adverse events should be defined in a personalized manner (Figs. 2, 3).
Among AS-AIH, ALF, and ACLF patients, estimation of the early biochemical response within 7–14 days is necessary (Fig. 2) [1,32]. In contrast, the midterm biochemical response of patients with nonsevere acute-onset AIH or chronic insidious AIH, even with cirrhosis, can be evaluated at 4–8 weeks (Fig. 3) [1]. Biochemical remission is followed by a histological remission of disease activity. Sustaining biochemical remission for a long term (>1 year from treatment initiation) is thereafter a surrogate for favorable overall long-term survival [52]. Decreasing values of VCTE are favorable, even for the regression of fibrosis.
The long-term outcome of patients with AIH has been shown to be improved with immunosuppressive treatment, both with corticosteroids alone and with a combination of a lower dose of corticosteroids and azathioprine (AZA) [53]; those regimens are consistently endorsed as a first-line treatment for AIH. The 2019 AASLD practice guidance and guidelines updated their recommended first-line treatment: either prednisone monotherapy (40–60 mg/day) or a combination of prednisone (20–40 mg/day) or budesonide (9 mg/day) and AZA (50–100 mg/day) [1]. The 2015 European Association for the Study of the Liver (EASL) guidelines propose 0.5–1 mg/kg/day predniso(lo)ne as the initial treatment, followed by a 50 mg/day AZA add-on [54]. The AASLD similarly indicates the appropriateness of a 2-week observation before the AZA is initiated, to confirm the patient’s steroid responsiveness and to evaluate his or her thiopurine-S methyl transferase (TPMT) status for the prevention of AZA-induced hepatitis. TPMT is an anabolizing enzyme for thiopurines, including AZA, and single nucleotide polymorphisms of TPMT genes that cause loss of enzymatic activity predispose patients, in particular European and African descendants, to thiopurine-related toxicity [1,55].
In Japan, AZA was finally approved for AIH treatment in 2018. Accordingly, the practice guidelines for AIH published by the Intractable Hepato-Biliary Diseases Study Group in Japan very recently added the recommendation to evaluate NUDT15 variant (but not TPMT) in patients who are to be treated with AZA, in order to exclude the possibility of thiopurine-induced early severe leukopenia and hair loss [55]. NUDT15 is a recently characterized nucleotide phosphatase that inactivates thiopurines. As the low- or intermediate activity diplotype was reported to be common in East Asian countries (22.6%) [55], the integration of NUDT15 variants in the dosing algorithm for AZA is regarded as most informative.
Concerning the relevance of the starting dose of predniso(lo)ne to ensure remission, a retrospective observational study from nine centers in five European countries was performed and the results revealed no significant difference in the rate of normalization of transaminases at 6 months between groups with a higher (≥0.5 mg/kg/day) and lower (<0.5 mg/kg/day) initial dose of predniso(lo)ne [56]. With the aid of AZA as a maintenance therapy in the majority of patients (>85%), an initial lower dose significantly decreased the unnecessary exposure to predniso(lo)ne in patients with AIH.
A synthetic steroid, i.e., budesonide has been shown to cause less systemic adverse effects, due to a 90% first-pass hepatic clearance rate. The AASLD investigated whether prednisone or predniso(lo)ne alone or in combination with AZA was superior to a combination of budesonide and AZA as the first-line treatment for patients with newly diagnosed AIH [1]. With an accompanying systemic review and meta-analysis, the AASLD demonstrated a higher rate of biochemical remission in the budesonide + AZA group compared to the prednisone + AZA group (odds ratio, 2.19; 95% CI, 1.30–3.67), and they described this finding as high-grade evidence [57]. Accordingly, the AASLD suggests budesonide in combination with AZA as a first-line therapy for child and adult AIH patients who do not have cirrhosis or acute severe AIH [1]; patients with cirrhosis are contraindicated for budesonide because portosystemic shunting may reduce the drug’s efficacy.
The combination of AZA and either predniso(lo)ne or budesonide is now regarded as the most standard first-line therapy in western countries. Prednisone monotherapy, on the other hand, is likely to be appropriate for patients including those with DIAIH-like injury in whom the treatment duration is expected to be <6 months [1].
As corticosteroids are still the mainstay of the first-line treatment of AIH, the maintenance of bone during treatment is needed to limit treatment-related osteoporosis in patients with ongoing risk factors [58]. Bone mineral densitometry should be completed at baseline in those patients with repeated check-up every 2–3 years and supplementation with elemental calcium (1,000–1,200 mg/day) and vitamin D (400–800 IU/day) are recommended for all patients on glucocorticoid therapy [1,59]. Simultaneous bisphosphonate therapy is indeed indicated for patients with documented osteoporosis [60]. The determination of serum levels of 25-hydroxyvitamine D at diagnosis is justifiable, because vitamin D insufficiency (≤29 ng/mL) occurs frequently in patients with AIH (68–81%) [61,62] and even severe deficiency (<20 ng/mL) was reported to be documented in 20% of patients [62].
The aims of second-line treatments for AIH are to manage refractoriness, incomplete biochemical response, and drug intolerance to first-line treatments (Figs. 2, 3). Anecdotally, second-line treatments have been performed with mycophenolate mofetil (MMF), calcineurin inhibitors (cyclosporin A, tacrolimus [TAC]), mercaptopurine, and biologics (e.g., infliximab).
MMF is a DNA synthesis inhibitor and is indicated for immunosuppression after organ transplant or lupus nephritis. In a meta-analysis, the combination of MMF + prednisone was shown to be the most widely prescribed second-line treatment, achieving histological remission in 89% of the patients [63]. A recent report confirmed the effectiveness of MMF as a second-line therapy for patients who have failed standard therapy; the rate of induction of biochemical remission was 60% [64].
The AASLD performed a systemic review to compare the efficacies of MMF and TAC for treatment failure or incomplete biochemical response in adults and children: the AASLD 2019 conditional recommendation with low certainty suggests the use of MMP or TAC to achieve and maintain biochemical remission [1].
During the course of maintenance therapy with corticosteroids/AZA, a substantial number of AIH patients spontaneously and asymptomatically experience biochemical exacerbation or recrudescence, i.e., an elevation of ALT coupled with or without an increase in IgG. The 2019 AASLD practice guidance and guidelines strictly define “relapse” as disease exacerbation that occurs after remission and drug withdrawal or by nonadherence [1]. Multiple relapses have been shown to be associated with worse outcomes [51], but the definitions of relapse in the literature differ from that issued by the AASLD, including the concept that biochemical remission may not have proceeded relapse. Following the AASLD rules regarding biochemical remission-induction with first-line or even second-line drugs could result in fewer exacerbations. Relapse after drug withdrawal (which usually occurs within 12 months) and exacerbation should be managed appropriately to induce re-(biochemical) remission with an increase in dosage or the reinstitution of immunosuppressive agents, or with the add-on of second-line drugs. In a case-control study, psychological stress was associated with relapse after drug taper-off or recrudescence [65].
If AIH is a curable disease, the cessation of immunosuppressive agents is desirable. Could the cure for AIH be diagnosed based on serum biochemistry and liver histology, or both? Is the cure achievable in specific subgroups of patients? The answers to these clinical questions involve the feasibility of treatment withdrawal and simultaneously pursuing the lowest risk of drug-induced complications.
Clinically, the duration and the degree of remission are the initial keys to the success of treatment withdrawal. A sustained biochemical remission of ≥2 years was proposed by the AASLD as the eligibility criterion for attempting a treatment withdrawal (Fig. 3) [1], in part because the inclusion of patients with only normalized ALT for 2 years resulted in almost universal relapse [66]. A further patient selection step should be included based on liver biochemistry, and/or on liver histology. Lower ALT and IgG values within the normal range were reported to be negative predictors of relapse; patients who achieved sustained remission for >1 year after drug withdrawal were all characterized by ALT values ≤0.5× ULN and IgG values ≤1,200 mg/dL [67]. Maintenance therapy before withdrawal was not associated with relapse: >80% of the patients were treated with AZA alone.
A single-center study demonstrated that only 10% of their patients were eligible for treatment withdrawal and 5% reached sustained remission without treatment [67], highlighting AIH as generally a chronic disease demanding life-long maintenance therapy. Stringent biochemical remission for >2 years along with sustained low values of VCTE measured with an appropriate cut-off, may identify the patients who are at low risk of decompensation even when relapse occurs after treatment withdrawal.
AIH with decompensated cirrhosis or ALF is indicated for LT. Among the listing of the Scientific Registry of Transplant Recipients (2002–2019) in the USA, 3.3% had AIH as the primary etiology [68]. In the trend analysis of the etiology of the Registry’s non-hepatocellular carcinoma LT listings, the rate of AIH during that period was stable.
In the prospective multicenter European Liver Transplant Registry (1998–2017), the overall survival of patients after AIH-LT was reported to be similar to that of patients after alcohol-related cirrhosis-LT, but worse than that after PBC-LT and PSC-LT [69]; the 5- and 10-year patient and graft survival rates after AIH-LT were 79.4% and 70.8% and 73.25% and 63.4%, respectively. Compared to all of the other groups, the AIH-LT patients were at higher risk for infections—especially lethal fungal infections resulting in death and graft loss [69]. In AIH, living donor transplantation provided worse survival than that by donated LT after brain death [69].
The appropriateness of long-term glucocorticoid therapy after LT remains a matter of debate, in part because acute, steroid-resistant, and chronic rejection occurred more frequently in adult AIH patients who underwent LT compared to patients with other liver diseases [70], and also because of the chance of recurrence after LT [71]. A systemic review and meta-analysis of continuous glucocorticoid therapy in AIH-LT patients by the AASLD suggests that a gradual cessation of glucocorticoids could be considered after LT, with very low certainty [1].
The topics not addressed in this review include genetics, potential therapeutics based on the current understanding of the immune-pathogenesis of AIH, the inequity of AIH disease management worldwide, and patient-reported outcomes highlighted by the health-related quality of life. For example, the marked disparity in the prevalence of cirrhosis around the world, exemplified by the very high rate in South Asia [72], should be evaluated based on determinations of the patients’ genetic backgrounds and managed by the standardization of diagnosis and treatment. Improvements are anticipated regarding the accessibility to the flowchart of AIH diagnosis, with special attention to the differential diagnosis from emerging pandemic NASH. At the same time, the health-related quality of life of AIH patients, which was reported to be severely impaired [73-75], must be evaluated for future improvement from the standpoint of personalized management including appropriate first-line therapy even with potential therapeutics, and by the prediction of the success of treatment withdrawal. Using a multifaceted approach, we hepatologists are encouraged to achieve AIH patients’ total wellness.
Figure 1.
A schematic model of the progression and transition of AIH. Acute-onset and acute exacerbation of AIH may resolve to chronic AIH, if not all. DILI, drug-induced liver injury; DIAIH, drug-induced autoimmune hepatitis; NAFLD, non-alcoholic fatty liver disease; AIH, autoimmune hepatitis; AS, acute severe; ALF, acute liver failure.
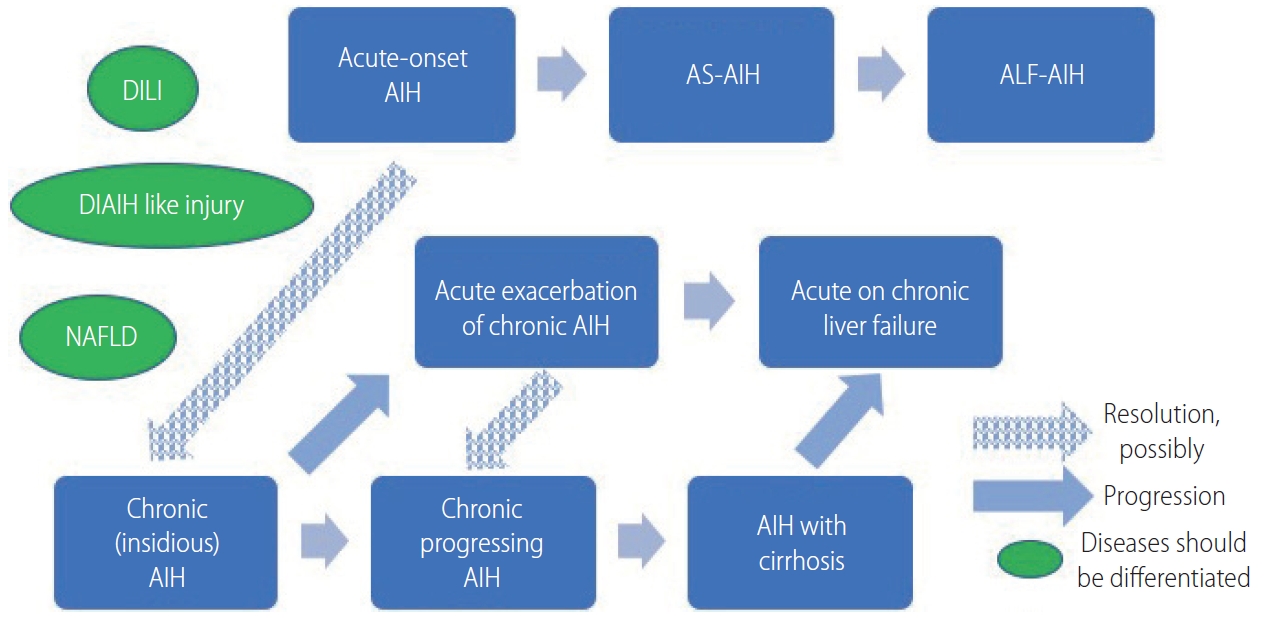
Figure 2.
The treatment response-guided management of AS-AIH. AS, acute severe; AIH, autoimmune hepatitis; MELD, model for end-stage liver disease; HE, hepatic encephalopathy; LT, liver transplantation; ALF, acute liver failure.

Figure 3.
The treatment response-guided management of AIH, excluding AS-AIH and ALF-AIH. AIH, autoimmune hepatitis; AS, acute severe; ALF, acute liver failure.
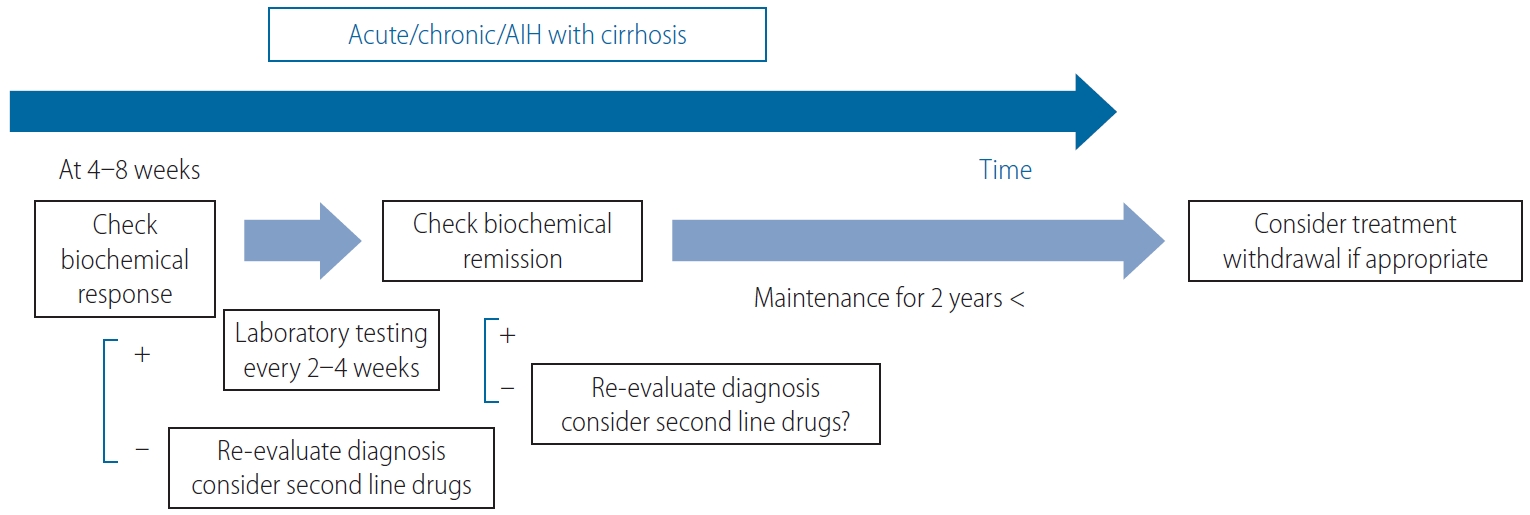
Table 1.
Definitions of proposed clinical subgroups of AIH and treatment outcomes
| Condition | Definition |
|---|---|
| Acute severe AIH | Jaundice, PT-INR ≥1.5, no encephalopathy; no previously diagnosed liver disease [1] |
| ALF-AIH | Jaundice, PT-INR ≥1.5, hepatic encephalopathy within 26 weeks of onset of illness; no previously diagnosed liver disease [1] |
| Biochemical remission | Normalization of serum AST, ALT, and IgG within ULN [1] |
| Incomplete response | Improvement of laboratory findings, but not to fulfill criteria for remission |
| Treatment failure | Worsening laboratory and histological findings under strict adherence to prescribed medication |
| Treatment intolerance | Inability to adhere to maintenance therapy due to drug-related side effects |
| Relapse | Exacerbation of disease activity after biochemical remission or nonadherence |
Abbreviations
AASLD
American Association of the Study of Liver Disease
ACLF
acute on chronic liver failure
AIH
autoimmune hepatitis
ALF
acute liver failure
ALT
alanine aminotransferase
AMAs
anti-mitochondrial antibodies
ANA
anti-nuclear antibodies
APRI
aspartate aminotransferase/platelet ratio index
AS
acute severe
AST
aspartate aminotransferase
AZA
azathioprine
CHC
chronic hepatitis C
CI
confidence interval
CT
computed tomography
DAA
direct-acting antiviral
DIAIH
drug-induced autoimmune hepatitis
DILI
drug-induced liver injury
EASL
European Association for the Study of the Liver
FIB-4
fibrosis-4
IAIHG
International Autoimmune Hepatitis Group
IgG
immunoglobulin G
INR
international normalized ratio
iv.
intravenous
LT
liver transplantation
M2BPGi
Mac-2 binding protein glycosylation isomer
MELD
model for end-stage liver disease
MMF
mycophenolate mofetil
NAFLD
non-alcoholic fatty liver disease
NASH
non-alcoholic steatohepatitis
PBC
primary biliary cholangitis
PSC
primary sclerosing cholangitis
PT
prothrombin time
TAC
tacrolimus
TPMT
thiopurine-S methyl transferase
ULN
upper limit of normal
VCTE
vibration-controlled transient elastography
REFERENCES
1. Mack CL, Adams D, Assis DN, Kerkar N, Manns MP, Mayo MJ, et al. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American Association for the Study of Liver Diseases. Hepatology 2020;72:671-722.


2. Kim BH, Choi HY, Ki M, Kim KA, Jang ES, Jeong SH. Population-based prevalence, incidence, and disease burden of autoimmune hepatitis in South Korea. PLoS One 2017;12:e0182391.



3. Takahashi A, Arinaga-Hino T, Ohira H, Torimura T, Zeniya M, Abe M, et al. Autoimmune hepatitis in Japan: trends in a nationwide survey. J Gastroenterol 2017;52:631-640.



4. Tanaka A, Mori M, Matsumoto K, Ohira H, Tazuma S, Takikawa H. Increase trend in the prevalence and male-to-female ratio of primary biliary cholangitis, autoimmune hepatitis, and primary sclerosing cholangitis in Japan. Hepatol Res 2019;49:881-889.


5. Lee YM, Teo EK, Ng TM, Khor C, Fock KM. Autoimmune hepatitis in Singapore: a rare syndrome affecting middle-aged women. J Gastroenterol Hepatol 2001;16:1384-1389.


6. Hurlburt KJ, McMahon BJ, Deubner H, Hsu-Trawinski B, Williams JL, Kowdley KV. Prevalence of autoimmune liver disease in Alaska natives. Am J Gastroenterol 2002;97:2402-2407.


7. Werner M, Prytz H, Ohlsson B, Almer S, Björnsson E, Bergquist A, et al. Epidemiology and the initial presentation of autoimmune hepatitis in Sweden: a nationwide study. Scand J Gastroenterol 2008;43:1232-1240.


8. Danielsson Borssén Å, Marschall HU, Bergquist A, Rorsman F, Weiland O, Kechagias S, et al. Epidemiology and causes of death in a Swedish cohort of patients with autoimmune hepatitis. Scand J Gastroenterol 2017;52:1022-1028.


9. Johnson PJ, McFarlane IG. Meeting report: International Autoimmune Hepatitis Group. Hepatology 1993;18:998-1005.


10. Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, et al. International Autoimmune Hepatitis Group report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol 1999;31:929-938.


11. Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology 2008;48:169-176.


12. Czaja AJ. Performance parameters of the diagnostic scoring systems for autoimmune hepatitis. Hepatology 2008;48:1540-1548.


13. Boberg KM, Chapman RW, Hirschfield GM, Lohse AW, Manns MP, Schrumpf E, et al. Overlap syndromes: the International Autoimmune Hepatitis Group (IAIHG) position statement on a controversial issue. J Hepatol 2011;54:374-385.


14. Yatsuji S, Hashimoto E, Kaneda H, Taniai M, Tokushige K, Shiratori K. Diagnosing autoimmune hepatitis in nonalcoholic fatty liver disease: is the International Autoimmune Hepatitis Group scoring system useful? J Gastroenterol 2005;40:1130-1138.



15. Gurung A, Assis DN, McCarty TR, Mitchell KA, Boyer JL, Jain D. Histologic features of autoimmune hepatitis: a critical appraisal. Hum Pathol 2018;82:51-60.


16. Tucker SM, Jonas MM, Perez-Atayde AR. Hyaline droplets in Kupffer cells: a novel diagnostic clue for autoimmune hepatitis. Am J Surg Pathol 2015;39:772-778.


17. Nguyen Canh H, Harada K, Ouchi H, Sato Y, Tsuneyama K, Kage M, et al. Acute presentation of autoimmune hepatitis: a multicentre study with detailed histological evaluation in a large cohort of patients. J Clin Pathol 2017;70:961-969.


18. Stravitz RT, Lefkowitch JH, Fontana RJ, Gershwin ME, Leung PS, Sterling RK, et al. Autoimmune acute liver failure: proposed clinical and histological criteria. Hepatology 2011;53:517-526.



19. De Luca-Johnson J, Wangensteen KJ, Hanson J, Krawitt E, Wilcox R. Natural history of patients presenting with autoimmune hepatitis and coincident nonalcoholic fatty liver disease. Dig Dis Sci 2016;61:2710-2720.




20. Takahashi A, Arinaga-Hino T, Ohira H, Abe K, Torimura T, Zeniya M, et al. Non-alcoholic fatty liver disease in patients with autoimmune hepatitis. JGH Open 2018;2:54-58.



21. Tapper EB, Lok AS. Use of liver imaging and biopsy in clinical practice. N Engl J Med 2017;377:756-768.


22. Wu S, Yang Z, Zhou J, Zeng N, He Z, Zhan S, et al. Systematic review: diagnostic accuracy of non-invasive tests for staging liver fibrosis in autoimmune hepatitis. Hepatol Int 2019;13:91-101.



23. Hartl J, Denzer U, Ehlken H, Zenouzi R, Peiseler M, Sebode M, et al. Transient elastography in autoimmune hepatitis: timing determines the impact of inflammation and fibrosis. J Hepatol 2016;65:769-775.


24. Yamasaki K, Tateyama M, Abiru S, Komori A, Nagaoka S, Saeki A, et al. Elevated serum levels of Wisteria floribunda agglutinin-positive human Mac-2 binding protein predict the development of hepatocellular carcinoma in hepatitis C patients. Hepatology 2014;60:1563-1570.



25. Migita K, Horai Y, Kozuru H, Koga T, Abiru S, Yamasaki K, et al. Serum cytokine profiles and Mac-2 binding protein glycosylation isomer (M2BPGi) level in patients with autoimmune hepatitis. Medicine (Baltimore) 2018;97:e13450.



27. Kim BH, Kim YJ, Jeong SH, Tak WY, Ahn SH, Lee YJ, et al. Clinical features of autoimmune hepatitis and comparison of two diagnostic criteria in Korea: a nationwide, multicenter study. J Gastroenterol Hepatol 2013;28:128-134.


28. Muratori P, Carbone M, Stangos G, Perini L, Lalanne C, Ronca V, et al. Clinical and prognostic implications of acute onset of autoimmune hepatitis: an Italian multicentre study. Dig Liver Dis 2018;50:698-702.


29. Joshita S, Yoshizawa K, Umemura T, Ohira H, Takahashi A, Harada K, et al. Clinical features of autoimmune hepatitis with acute presentation: a Japanese nationwide survey. J Gastroenterol 2018;53:1079-1088.



30. Yeoman AD, Westbrook RH, Zen Y, Bernal W, Al-Chalabi T, Wendon JA, et al. Prognosis of acute severe autoimmune hepatitis (ASAIH): the role of corticosteroids in modifying outcome. J Hepatol 2014;61:876-882.


31. Moenne-Loccoz R, Severac F, Baumert TF, Habersetzer F. Usefulness of corticosteroids as first-line therapy in patients with acute severe autoimmune hepatitis. J Hepatol 2016;65:444-446.


32. Rahim MN, Liberal R, Miquel R, Heaton ND, Heneghan MA. Acute severe autoimmune hepatitis: corticosteroids or liver ttransplantation? Liver Transpl 2019;25:946-959.


33. Zachou K, Arvaniti P, Azariadis K, Lygoura V, Gatselis NK, Lyberopoulou A, et al. Prompt initiation of high-dose i.v. corticosteroids seems to prevent progression to liver failure in patients with original acute severe autoimmune hepatitis. Hepatol Res 2019;49:96-104.


34. Yasui S, Fujiwara K, Okitsu K, Yonemitsu Y, Ito H, Yokosuka O. Importance of computed tomography imaging features for the diagnosis of autoimmune acute liver failure. Hepatol Res 2012;42:42-50.


35. Zabron A, Quaglia A, Fatourou E, Peddu P, Lewis D, Heneghan M, et al. Clinical and prognostic associations of liver volume determined by computed tomography in acute liver failure. Liver Int 2018;38:1592-1601.


36. Karkhanis J, Verna EC, Chang MS, Stravitz RT, Schilsky M, Lee WM, et al. Steroid use in acute liver failure. Hepatology 2014;59:612-621.



37. Anand L, Choudhury A, Bihari C, Sharma BC, Kumar M, Maiwall R, et al. Flare of autoimmune hepatitis causing acute on chronic liver failure: diagnosis and response to corticosteroid therapy. Hepatology 2019;70:587-596.


38. Weber S, Benesic A, Rotter I, Gerbes AL. Early ALT response to corticosteroid treatment distinguishes idiosyncratic drug-induced liver injury from autoimmune hepatitis. Liver Int 2019;39:1906-1917.


40. Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, et al. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology 2015;148:1340-1352 e7.



41. de Boer YS, Kosinski AS, Urban TJ, Zhao Z, Long N, Chalasani N, et al. Features of autoimmune hepatitis in patients with drug-induced liver injury. Clin Gastroenterol Hepatol 2017;15:103-112 e2.



42. Robles-Diaz M, Lucena MI, Kaplowitz N, Stephens C, Medina-Cáliz I, González-Jimenez A, et al. Use of Hy’s law and a new composite algorithm to predict acute liver failure in patients with drug-induced liver injury. Gastroenterology 2014;147:109-118 e5.


43. Björnsson E, Talwalkar J, Treeprasertsuk S, Kamath PS, Takahashi N, Sanderson S, et al. Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology 2010;51:2040-2048.


44. Zen Y, Yeh MM. Hepatotoxicity of immune checkpoint inhibitors: a histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug-induced liver injury. Mod Pathol 2018;31:965-973.



45. Nishida N, Kudo M. Liver damage related to immune checkpoint inhibitors. Hepatol Int 2019;13:248-252.



46. Gatselis NK, Dalekos GN. Molecular diagnostic testing for primary biliary cholangitis. Expert Rev Mol Diagn 2016;16:1001-1010.


47. Chazouillères O, Wendum D, Serfaty L, Montembault S, Rosmorduc O, Poupon R. Primary biliary cirrhosis-autoimmune hepatitis overlap syndrome: clinical features and response to therapy. Hepatology 1998;28:296-301.


48. Bonder A, Retana A, Winston DM, Leung J, Kaplan MM. Prevalence of primary biliary cirrhosis-autoimmune hepatitis overlap syndrome. Clin Gastroenterol Hepatol 2011;9:609-612.


49. Czaja AJ. Cholestatic phenotypes of autoimmune hepatitis. Clin Gastroenterol Hepatol 2014;12:1430-1438.


50. Simoes CC, Saldarriaga OA, Utay NS, Stueck AE, Merwat SK, Merwat SN, et al. Direct-acting antiviral treatment of patients with hepatitis C resolves serologic and histopathologic features of autoimmune hepatitis. Hepatol Commun 2019;3:1113-1123.



51. Yoshizawa K, Matsumoto A, Ichijo T, Umemura T, Joshita S, Komatsu M, et al. Long-term outcome of Japanese patients with type 1 autoimmune hepatitis. Hepatology 2012;56:668-676.


52. Choi J, Choi GH, Lee D, Shim JH, Lim YS, Lee HC, et al. Long-term clinical outcomes in patients with autoimmune hepatitis according to treatment response in Asian country. Liver Int 2019;39:985-994.


53. Lamers MM, van Oijen MG, Pronk M, Drenth JP. Treatment options for autoimmune hepatitis: a systematic review of randomized controlled trials. J Hepatol 2010;53:191-198.


54. European Association for the Study of the Liver. EASL clinical practice guidelines: autoimmune hepatitis. J Hepatol 2015;63:971-1004.


55. Moriyama T, Nishii R, Perez-Andreu V, Yang W, Klussmann FA, Zhao X, et al. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet 2016;48:367-373.




56. Pape S, Gevers TJG, Belias M, Mustafajev IF, Vrolijk JM, van Hoek B, et al. Predniso(lo)ne dosage and chance of remission in patients with autoimmune hepatitis. Clin Gastroenterol Hepatol 2019;17:2068-2075 e2.


57. Vierling JM, Kerkar N, Czaja AJ, Mack CL, Adams D, Assis DN, et al. Immunosuppressive treatment regimens in autoimmune hepatitis: systematic reviews and meta-analyses supporting American Association for the Study of Liver Diseases guidelines. Hepatology 2020;72:753-769.


58. American Gastroenterological Association. American Gastroenterological Association medical position statement: osteoporosis in hepatic disorders. Gastroenterology 2003;125:937-940.


59. Long MD, Thiny MT, Sandler RS, Gangarosa LM. Bone health in a tertiary-care gastroenterology and hepatology population. Dig Dis Sci 2010;55:2263-2269.




60. Suzuki Y, Nawata H, Soen S, Fujiwara S, Nakayama H, Tanaka I, et al. Guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for Bone and Mineral Research: 2014 update. J Bone Miner Metab 2014;32:337-350.



61. Efe C, Kav T, Aydin C, Cengiz M, Imga NN, Purnak T, et al. Low serum vitamin D levels are associated with severe histological features and poor response to therapy in patients with autoimmune hepatitis. Dig Dis Sci 2014;59:3035-3042.



62. Ebadi M, Bhanji RA, Mazurak VC, Lytvyak E, Mason A, Czaja AJ, et al. Severe vitamin D deficiency is a prognostic biomarker in autoimmune hepatitis. Aliment Pharmacol Ther 2019;49:173-182.


63. De Lemos-Bonotto M, Valle-Tovo C, Costabeber AM, Mattos AA, Azeredo-da-Silva ALF. A systematic review and meta-analysis of second-line immunosuppressants for autoimmune hepatitis treatment. Eur J Gastroenterol Hepatol 2018;30:212-216.


64. Nicoll AJ, Roberts SK, Lim R, Mitchell J, Weltman M, George J, et al. Beneficial response to mycophenolate mofetil by patients with autoimmune hepatitis who have failed standard therapy, is predicted by older age and lower immunoglobulin G and INR levels. Aliment Pharmacol Ther 2019;49:1314-1322.


65. Srivastava S, Boyer JL. Psychological stress is associated with relapse in type 1 autoimmune hepatitis. Liver Int 2010;30:1439-1447.



66. van Gerven NM, Verwer BJ, Witte BI, van Hoek B, Coenraad MJ, van Erpecum KJ, et al. Relapse is almost universal after withdrawal of immunosuppressive medication in patients with autoimmune hepatitis in remission. J Hepatol 2013;58:141-147.


67. Hartl J, Ehlken H, Weiler-Normann C, Sebode M, Kreuels B, Pannicke N, et al. Patient selection based on treatment duration and liver biochemistry increases success rates after treatment withdrawal in autoimmune hepatitis. J Hepatol 2015;62:642-646.


68. Younossi ZM, Stepanova M, Ong J, Trimble G, AlQahtani S, Younossi I, et al. Nonalcoholic steatohepatitis is the most rapidly increasing indication for liver transplantation in the United States. Clin Gastroenterol Hepatol 2020 Jun 9;doi: 10.1016/j.cgh.2020.05.064.

69. Heinemann M, Adam R, Berenguer M, Mirza D, Malek-Hosseini SA, O’Grady JG, et al. Longterm survival after liver transplantation for autoimmune hepatitis: results from the European Liver Transplant Registry. Liver Transpl 2020;26:866-877.


70. Hayashi M, Keeffe EB, Krams SM, Martinez OM, Ojogho ON, So SK, et al. Allograft rejection after liver transplantation for autoimmune liver diseases. Liver Transpl Surg 1998;4:208-214.


71. Milkiewicz P, Gunson B, Saksena S, Hathaway M, Hubscher SG, Elias E. Increased incidence of chronic rejection in adult patients transplanted for autoimmune hepatitis: assessment of risk factors. Transplantation 2000;70:477-480.


72. Tanaka A, Ma X, Yokosuka O, Weltman M, You H, Amarapurkar DN, et al. Autoimmune liver diseases in the Asia-Pacific region: proceedings of APASL symposium on AIH and PBC 2016. Hepatol Int 2016;10:909-915.



73. Takahashi A, Moriya K, Ohira H, Arinaga-Hino T, Zeniya M, Torimura T, et al. Health-related quality of life in patients with autoimmune hepatitis: a questionnaire survey. PLoS One 2018;13:e0204772.



- TOOLS
-
METRICS

- ORCID iDs
-
Atsumasa Komori

https://orcid.org/0000-0002-2607-9381 - Related articles
-
Recent advances in the management of hepatocellular carcinoma2024 January;30(1)
KASL clinical practice guidelines for management of autoimmune hepatitis 20222023 July;29(3)
The role of different viral biomarkers on the management of chronic hepatitis B2023 April;29(2)
Epidemiology and updated management for autoimmune liver disease2023 January;29(1)
KASL clinical practice guidelines for management of chronic hepatitis B2022 April;28(2)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



