| Clin Mol Hepatol > Volume 28(3); 2022 > Article |
|
See the commentary-article "Association of nonalcoholic fatty liver disease with incident dementia later in life among elderly adults" on page 481.
ABSTRACT
Background/Aims
Accumulating evidence suggests a link between non-alcoholic fatty liver disease (NAFLD) and brain health. However, population-based evidence on the association between NAFLD and dementia remains unclear. This study was conducted to determine the association between NAFLD and incident dementia.
Methods
The study population included 608,994 adults aged Ōēź60 years who underwent health examinations between 2009 and 2010. Data were collected from the Korean National Health Insurance Service database. NAFLD was assessed using the fatty liver index (FLI). A Cox proportional hazards regression model was used to determine the association between NAFLD and dementia.
Results
During the 6,495,352 person-years of follow-up, 48,538 participants (8.0%) developed incident dementia. The participants were classified into low (FLI <30), intermediate (FLI Ōēź30 and <60), and high (FLI Ōēź60) groups. In the overall study population, the FLI groups were associated with a risk of dementia (P for trend <0.001). After propensity score matching, a low FLI was associated with a reduced risk of dementia (adjusted hazard ration [aHR], 0.96; 95% confidence interval [CI], 0.93ŌĆō0.98; P=0.002), whereas a high FLI (NAFLD) was associated with an increased risk of dementia (aHR, 1.05; 95% CI, 1.02ŌĆō1.08; P=0.001). A higher risk of dementia in the high FLI group than in the intermediate FLI group was attributed to AlzheimerŌĆÖs disease (aHR, 1.04; 95% CI, 1.01ŌĆō1.07; P=0.004) rather than vascular dementia (aHR, 0.94; 95% CI, 0.75ŌĆō1.18; P=0.602).
Graphical Abstract
Approximately 50 million people suffer from dementia worldwide, and the number of elderly individuals with dementia continues to increase in the context of global population aging, placing a significant burden on the healthcare system [1-3]. Non-alcoholic fatty liver disease (NAFLD) is also increasing in prevalence as a representative non-communicable disease of the liver, affecting up to a quarter of the adult population in parallel with a global epidemic of obesity and metabolic syndrome [4]. Minimizing exposure to modifiable risk factors for dementia has been reported to reduce the incidence of dementia in several cohort studies [5-7]. In particular, a population-based study suggested that regular physical activity and management of cardiovascular risk factors may reduce the risk of cognitive decline and dementia [8]. Likewise, the elucidation and management of risk factors are important in reducing the incidence and burden of dementia.
Recent studies have yielded inconsistent results regarding the relationship between NAFLD and dementia. According to a previous National Health and Nutrition Examination Survey study, NAFLD was associated with cognitive impairment in the general USA population, independent of cardiovascular disease and its risk factors [9]. In contrast, a German cohort study demonstrated that neither the incidence of overall dementia, nor that of vascular dementia, was associated with NAFLD [10]. According to a Swedish cohort study, NAFLD itself was not associated with incident dementia; however, liver histology, especially fibrosis stage, could improve the predictive performance of dementia risk [11]. In addition, the Framingham study suggested that the presence of NAFLD was not associated with cognitive function, but the NAFLD fibrosis score (NFS) could predict cognitive impairment in patients with NAFLD [12]. Conversely, an Italian study demonstrated that NFS was not a significant risk factor for dementia [13]. Given the contradictory results, further larger-scale population-based studies that explore the potential impact of NAFLD on the risk of dementia are warranted. This study investigated the association of NAFLD with the risk of incident dementia, including AlzheimerŌĆÖs disease and vascular dementia, based on the fatty liver index (FLI).
Detailed information regarding the validity and design of the Korean National Health Insurance Service (NHIS) is described in a previous study [14]. Briefly, the NHIS is an insurance system established under the Ministry of Health and Welfare, which covers approximately 97% of the Korean population. The NHIS collects demographic characteristics, health screening results, healthcare and treatment, drug prescription, and questionnaire-based behavioral characteristics, and carries out quality control before providing data for research purposes.
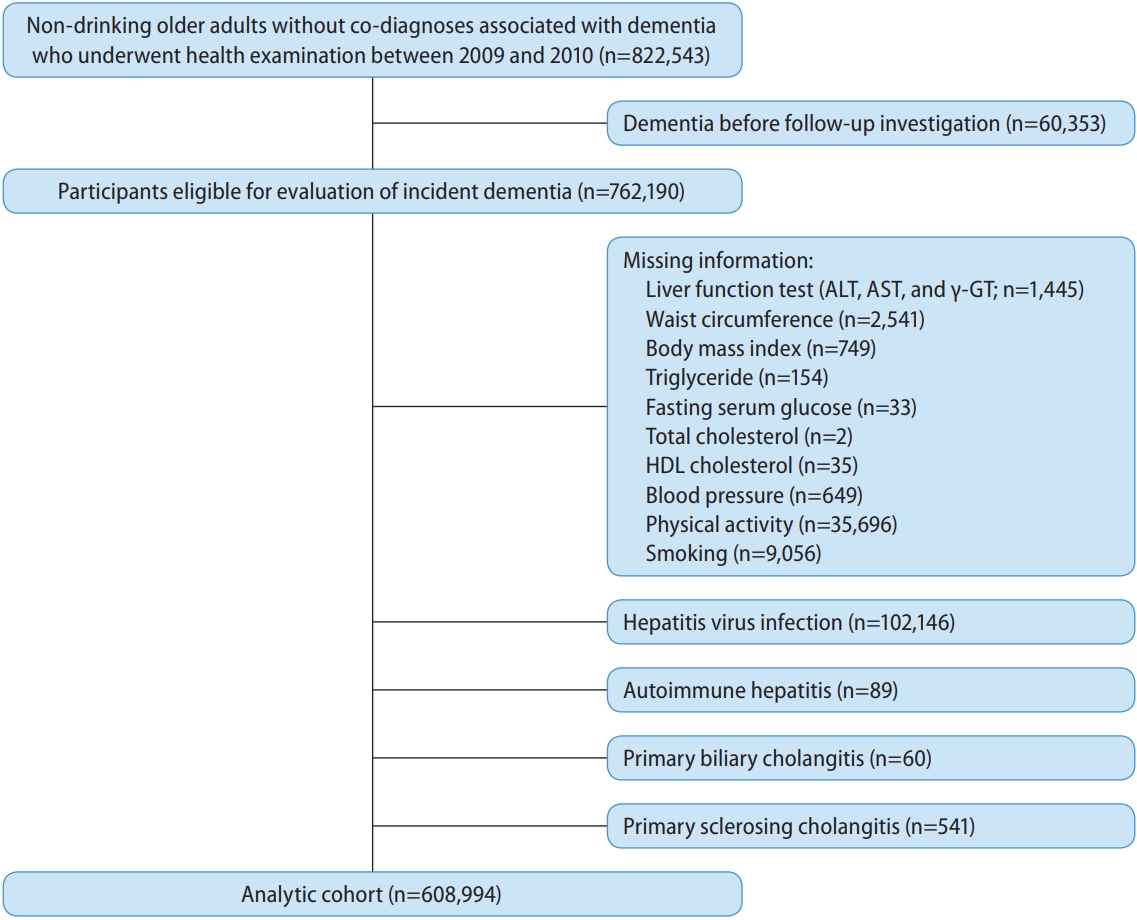
This study used data from the nationwide Korean NHIS database. There were 3,269,657 older adults aged Ōēź60 years who underwent health examinations between 2009 and 2010. Participants with a history of ischemic heart disease (International Classification of Diseases tenth revision [ICD-10], I20-I25; n=321,377), arterial hypertension (ICD-10, I10; n=970,856), heart failure (ICD-10, I50; n=45,753), renal failure (ICD-10, N18 and N19; n=3,221), stroke and transient ischemic attack (ICD-10, I60-I64 and G45; n=79,228), intracranial injury (ICD-10, S06; n=23,224), epilepsy (ICD-10, G40 and G41; n=6,345), ParkinsonŌĆÖs disease (ICD-10, G20 and G21; n=4,990), osteoporosis (ICD-10, M80 and M81; n=218,147), and depression (ICD-10, F32 and F33; n=33,848), before the follow-up investigation of dementia, were excluded. In addition, those with missing information on alcohol consumption (n=12,303) and those with alcohol consumption Ōēź1 times/week (n=727,822) were excluded. Among the remaining non-drinking older adults (n=822,543), participants with a history of dementia (n=60,353) prior to the follow-up investigation were also excluded from analysis. In addition, participants with missing information on the evaluation of the FLI, adjustment analysis, and stratified analysis, and those with chronic viral hepatitis infection (ICD-10, B18; n=102,146), autoimmune hepatitis (ICD-10, K754; n=89), primary biliary cholangitis (ICD-10, K743; n=60), and primary sclerosing cholangitis (ICD-10, K830; n=541) before the follow-up investigation were excluded from the analysis. None of the participants had WilsonŌĆÖs disease (ICD-10, E8301) or hemochromatosis (ICD-10, E8311) before enrollment. The final analytic cohort consisted of 608,994 participants (Fig. 1). This study was conducted in accordance with the Declaration of Helsinki and the STROBE guidelines. The Institutional Review Board of Seoul National University approved this study (E-1803-045-928). The requirement for informed consent was waived because the NHIS database provided anonymized data in accordance with the Personal Data Protection Act guidelines.
In the present study, dementia was operationally defined based on the ICD-10 codes F00, F01, F02, F03, and G30, along with dementia-associated medication use, including donepezil, galantamine, rivastigmine, and memantine. AlzheimerŌĆÖs disease was diagnosed when a participant had ICD-10 codes F00 and G30, whereas vascular dementia was diagnosed using the ICD-10 code F01 on the basis of the use of dementia-associated medications. All participants were followed from the date of health examination to the date of incident dementia, death, or December 31, 2020.
NAFLD was defined using the FLI, which was calculated using the following formula:
F L I = 1 ( 1 + exp ( ŌłÆ x ) ) ├Ś 100 x = 0.953 ├Ś log e ( s e r u m ŌĆē t r i g l y c e r i d e s ) + 0.139 ├Ś b o d y ┬Ā m a s s ┬Ā i n d e x ┬Ā B M I + 0.718 ┬Ā ├Ś ┬Ā l o g e ┬Ā s e r u m ┬Ā ╬│ ŌłÆ g l u t a m y l ┬Ā t r a n s p e p t i d a s e ┬Ā + 0.053 ┬Ā ├Ś ┬Ā w a i s t ┬Ā c i r c u m f e r e n c e ┬Ā ŌłÆ ┬Ā 15.745
Low and high FLIs were defined using the dual cutoffs of FLI (<30 and Ōēź60, respectively). The FLI is considered an acceptable alternative to imaging modalities according to the European Clinical Practice Guidelines [16]. In the Korean population, the FLI was validated with an area under the curve value of 0.87 in a receiver operating characteristic curve [17].
The National Cholesterol Education Program Adult Treatment Panel III was adopted to define metabolic syndrome as when three or more of the following criteria were met: waist circumference Ōēź90 cm for men and Ōēź80 cm for women, systolic blood pressure Ōēź130 or diastolic blood pressure Ōēź85 mmHg, triglyceride level Ōēź150 mg/dL, high-density lipoprotein cholesterol Ōēż40 mg/dL for men or Ōēż50 mg/dL for women, and fasting serum glucose (FSG) Ōēź100 mg/dL [18].
The following covariates were considered key variables for multivariate analyses: age (continuous; years), sex (categorical; men and women), household income (categorical; upper half and lower half), BMI (continuous; kg/m2), systolic blood pressure (continuous; mmHg), FSG (continuous; mg/dL), smoking (categorical; never, previous, and current), moderate-to-vigorous physical activity (categorical; Ōēż2, 3ŌĆō4, and Ōēź5 times/week), and Charlson comorbidity index (CCI; continuous). CCI was calculated as described in a previous study [19].
Categorical and continuous variables are presented as number (%) and median (interquartile range [IQR]), respectively. The Cox proportional hazards model was adopted to evaluate adjusted hazard ratios (aHRs) with 95% confidence intervals (CIs). The following models were analyzed to estimate the risk of incident dementia: model A, adjusted for age, sex, and BMI; model B, adjusted for age, sex, BMI, household income, systolic blood pressure, and FSG; and model C, adjusted for smoking, moderate-to-vigorous physical activity, and CCI in addition to factors included in model B.
Among the key variables, only independent predictive factors for dementia that were significant in the multivariate Cox regression analysis were selected as covariates for propensity score matching (PSM) to reduce confounding effects. PSM was conducted against the intermediate FLI group for both low and high FLI groups. A caliper of width equal to 0.2 of the standard deviation of the logit of the propensity score was used for 1:1 matching of subjects between the different FLI groups. The number of participants after PSM was 144,299 for low FLI and 145,799 for high FLI. The matched proportions of participants with low and high FLI were 75.0% and 54.2%, respectively.
Sensitivity analyses were performed after washing out the selected latent periods by excluding participants with dementia within the defined selected periods. Age, sex, BMI, hypertension, diabetes mellitus, dyslipidemia, smoking, physical activity, CCI, and metabolic syndrome were considered for stratified analyses to evaluate the interaction with FLI. The supremum test was performed to test the proportional hazards assumption in the Cox model. Statistical significance was set at P<0.05. All statistical analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute, Cary, NC, USA).
Table 1 presents the baseline characteristics of the study participants in the Korean NHIS cohort. Study participants included 192,874 men (31.6%) and 416,810 women (68.4%) with a median age of 65 years (IQR, 62ŌĆō69). More than half of the participants (n=374,765; 61.5%) belonged to the upper half of their household income. The median BMI and waist circumference were 23.6 kg/m2 and 81 cm, respectively. The majority of the participants had never smoked (n=533,722; 87.5%). In addition, hypertension, diabetes mellitus, and dyslipidemia were present in 82,603 (13.5%), 52,594 (8.6%), and 145,015 (23.8%) of participants, respectively. According to the FLI category, the participants were classified into the low (FLI <30; fatty liver rule-out; n=193,897), intermediate (FLI Ōēź30 and <60; n=145,814), and high (FLI Ōēź60; fatty liver rule-in; n=269,441) groups. The participants in the high FLI group were more likely to be men with higher levels of BMI, waist circumference, blood pressure, total cholesterol, and FSG.
During the 6,495,352 person-years of follow-up, 48,538 participants (8.0%) developed incident dementia. In addition, 46,852 (7.7%) and 688 (0.1%) participants developed AlzheimerŌĆÖs disease and vascular dementia, respectively (Table 2). In the fully adjusted model (model C), the risk of dementia was associated with FLI groups (P for trend <0.001), and a low FLI was associated with a decreased dementia risk (aHR, 0.97; 95% CI, 0.94ŌĆō0.99) compared to the intermediate group. AlzheimerŌĆÖs disease was significantly associated with the FLI group (P for trend=0.004), whereas vascular dementia was not associated with the FLI group (P for trend=0.117). Sensitivity analyses, after excluding latent periods for the development of dementia, demonstrated similar results to the primary findings (Supplementary Table 1).
We sought to determine whether a high FLI was associated with an increased dementia risk after PSM. Multivariate Cox regression analysis of the key variables included in the adjustments identified age, sex, BMI, household income, systolic blood pressure, FSG, smoking, moderate-to-vigorous physical activity, and CCI as independent factors associated with dementia risk (Supplementary Table 2). The logistic regression analysis results for PSM are shown in Supplementary Table 3. The descriptive statistics of the participants after PSM for the intermediate-and low-FLI groups are presented in Supplementary Table 4. Descriptive characteristics after PSM of subjects with a high FLI versus those with an intermediate FLI are shown in Supplementary Table 5.
After PSM, a low FLI was associated with a lower dementia risk (aHR, 0.96; 95% CI, 0.93ŌĆō0.98; P=0.002) in the final adjustment model (Table 3). In addition, a low FLI was associated with a lower risk of AlzheimerŌĆÖs disease (aHR, 0.96; 95% CI, 0.93ŌĆō0.99; P=0.005) but not with vascular dementia (aHR, 0.84; 95% CI, 0.66ŌĆō1.07; P=0.157) in the final adjustment model. In the matched cohort of both the intermediate and high FLI groups, a high FLI was associated with a higher dementia risk (aHR, 1.05; 95% CI, 1.02ŌĆō1.08; P=0.001). A significant association between high FLI and higher dementia risk was attributed to the increased risk of AlzheimerŌĆÖs disease in the high FLI group (aHR, 1.04; 95% CI, 1.01ŌĆō1.07; P=0.004) but not to vascular dementia (aHR, 0.94; 95% CI, 0.75ŌĆō1.18; P=0.602) in the final adjustment model.
After stratification of participants, a significant interaction was found between sex, hypertension, and dyslipidemia (Supplementary Table 6). A low FLI was associated with a decreased dementia risk in any age, sex, BMI <25 kg/m2, hypertension, no diabetes mellitus, no dyslipidemia, never smoking, and moderate-to-vigorous physical activity Ōēż2 times/ week. According to metabolic health, a low FLI showed a lower dementia risk in participants without metabolic syndrome and normal waist circumference, blood pressure, high-density lipoprotein cholesterol, and FSG.
Stratified analyses of the high-and intermediate-FLI groups are shown in Supplementary Table 7. No significant interactions were found between the selected variables used for stratification. A high FLI was associated with a higher risk of dementia among older adults, women, both BMI, no hypertension, no type 2 diabetes, no dyslipidemia, never and current smokers, moderate-to-vigorous physical activity Ōēż2 times/week and Ōēź5 times/week, CCI=1, no metabolic syndrome, normal waist circumference, abnormal blood pressure, normal triglyceride, normal high-density lipoprotein cholesterol, and normal FSG subgroups, as compared to an intermediate FLI.
The global epidemic of obesity has fueled the rapidly increasing burden of NAFLD, which has become a leading cause of end-stage liver diseases, hepatocellular carcinoma, and cardiometabolic diseases [20]. In the present study, FLI as a proxy for NAFLD was significantly associated with the risk of incident dementia. A significant association between high FLI and overall incident dementia attributable to AlzheimerŌĆÖs disease was found after PSM. Therefore, the management of NAFLD may reduce the disease burden related to dementia. In addition, exploring the underlying mechanisms linking NAFLD to incident dementia may provide new insights into preventive and therapeutic strategies against the development and progression of dementia.
Weinstein et al. [21] examined the relationship between NAFLD and total brain volume in 906 subjects enrolled in the Framingham offspring cohort. There were no significant associations between white matter hyperintensities and hippocampal volume, but they found a significant association with total brain volume. Even after adjustment for the covariates, patients with NAFLD had smaller-than-normal brains for their age, which can be seen as a pathologic acceleration of the brain aging process. This finding was most striking among the youngest subjects, accounting for about a 7-year advance in brain aging for those younger than 60 years. Taken together, the contribution of fatty liver to dementia risk may be due to its biological effect on brain aging.
A growing body of research has linked insulin resistance to several neurodegenerative mechanisms of AlzheimerŌĆÖs disease, including oxidative stress, mitochondrial dysfunction, and chronic liver inflammation, via dysregulated insulin/insulin-like growth factor 1 signaling with accompanying impairments in signal transduction and gene expression [22-24]. A network clustering analysis demonstrated that 189 genes were shared between AlzheimerŌĆÖs disease and NAFLD [25]. The identified main pathways contributing to both AlzheimerŌĆÖs disease and NAFLD included carbohydrate metabolism, fatty acid metabolism, and interleukin-17 signaling pathways.
NAFLD may also increase amyloid burden and aggravate AlzheimerŌĆÖs pathology. This contribution can be largely attributed to an imbalance in peripheral amyloid-╬▓ (A╬▓) clearance as a result of a reduction in low-density lipoprotein receptor-related protein 1 (LRP-1) levels that are highly expressed in hepatocytes under physiological conditions [26]. Liver dysfunction is accompanied by low expression of hepatic LRP-1 and high levels of circulating A╬▓, suggesting that A╬▓ clearance decreases due to low hepatic LRP-1 expression. Alternatively, insulin promotes LRP-1 translocation to the cell membrane in hepatocytes, favoring A╬▓ clearance [27]. The stimulation of LRP-1-mediated liver uptake indeed ameliorates cognitive dysfunction and decreases A╬▓ aggregation in the brains of AlzheimerŌĆÖs disease transgenic mice [28]. These features may also disrupt the blood-brain barrier and contribute to a vicious cycle.
AlzheimerŌĆÖs disease is an irreversible neurodegenerative disease in which neuroinflammation plays a critical role [29]. A preclinical study demonstrated that NAFLD-induced chronic liver inflammation contributes to the pathogenesis of AlzheimerŌĆÖs disease by inducing neurodegeneration in a genetic predisposition-absent setting [24]. They showed that NAFLD induced by a high-fat diet (HFD) promotes the development of AlzheimerŌĆÖs disease in mice. Brains of HFD-fed mice revealed increased levels of neuroinflammation with higher levels of pro-inflammatory cytokines, toll-like receptors, and microgliosis, which were accompanied by increased plaque formation in AlzheimerŌĆÖs disease transgenic mice. Furthermore, lipocalin-2 (Lcn2) is an adipokine exclusively produced in the liver and circulates throughout the body among individuals with nonalcoholic steatohepatitis (NASH) [30]. Recently, a murine model of NASH revealed that high levels of Lcn2 circulating in the bloodstream can activate a number of pro-inflammatory processes in the brain. The study also suggested that Lcn2 induces a weakening of the blood-brain barrier, which subsequently increases the expression of inflammatory molecules in brain endothelial cells [31].
The adaptive immune response has been found to contribute to the development of AlzheimerŌĆÖs disease [32]. Adaptive immune responses were noticeable in the blood and cerebrospinal fluid collected from patients with AlzheimerŌĆÖs disease, with clonal antigen-experienced CD8+ T cells patrolling the intrathecal space of the brain and are affected by age-associated neurodegeneration [33]. The evolution of NAFLD to NASH is accompanied by an increased frequency of intrahepatic cytotoxic CD8+ T cells [34]. These cells were recruited in response to signals modulated by interferon-╬▒, and exacerbated insulin resistance and glucose intolerance in the livers of HFD-fed mice [35]. In addition, mice lacking CD8+ T cells and natural killer T (NKT) cells were protected from steatosis when fed a choline-deficient HFD, which was related to a reduction in soluble mediators, such as lymphotoxin-like inducible protein that competes with glycoprotein D for binding herpes virus entry mediator on T cells and lymphotoxin, released by CD8+ T cells and NKT cells [36]. Furthermore, the selective ablation of CD8+ T cells demonstrated effectiveness in the amelioration of steatohepatitis in mice fed a high-fat and high-carbohydrate diet, indicating a pathogenic role of adaptive immunity in the development of NASH [37]. These findings suggest a close relationship between intrahepatic adaptive immunity and adaptive immune response within the brain, which awaits further experimental validation.
Recent studies have suggested that advanced fibrosis may impact the risk of cognitive dysfunction and incident dementia [11,12]. Although the exact pathogenic mechanism for cognitive impairment in individuals with NASH and advanced fibrosis remains unclear, neuroinflammation and changes in brain-derived neurotrophic factor levels may interact on the same causal pathway of liver fibrosis and cognitive dysfunction [38-40]. We speculate that liver fibrosis may result in the overexpression of pro-inflammatory cytokines, leading to a reduction in brain-derived neurotrophic factor levels, and ultimately to cognitive impairment. Further studies are required to define the association between advanced fibrosis and the risk of dementia. Stratified analyses revealed that current smokers with low FLI had no significant beneficial effects on dementia risk compared to those with intermediate FLI, but high FLI subjects showed a significant increase in dementia risk. In addition, dementia risk was significantly reduced only in participants with a lower BMI. These results suggest that modification of lifestyle behaviors (i.e., smoking cessation and weight loss) should be accompanied in the evaluation of NAFLD-associated dementia risk.
This study had some limitations that need to be considered. First, NAFLD was operationally defined using the FLI. Further larger-scale validation based on radiologic or pathologic confirmation of NAFLD may strengthen the intrinsic association between fatty liver and dementia. However, FLI evaluation allows the identification of the low FLI group, which is difficult to implement using conventional approaches in a real-world setting. Second, our study population consisted only of an East Asian population. Considering ethnicity-related differences in BMI and waist circumference, our results require further validation in other ethnic populations. Third, although additional functional studies based on gene expression and biological characteristics were not conducted in the present study, our findings merit further mechanistic investigation. In addition, despite the exclusion of participants with alcohol consumption with a frequency of Ōēź1 times/week, we might have failed to identify those with heavy alcohol consumption at an undetectable frequency (<1 time/week). Lastly, not all potential covariates that may be associated with dementia risk, such as education level, could be included in the adjustment. Nevertheless, our study is the first large-scale population-based study to explore the association of fatty liver with incident dementia at a nationwide level.
In conclusion, NAFLD, defined using the FLI, is independently associated with a higher risk of incident dementia attributable to AlzheimerŌĆÖs disease, whereas a low FLI was associated with a lower risk of dementia. Although additional research is warranted to further clarify the underlying mechanism, accumulating evidence of the link between fatty liver and brain health, such as an epidemiologic association, may be mediated by the complex interplay between metabolism and vascular function in the liver.
ACKNOWLEDGMENTS
Won Kim received a National Research Foundation of Korea (NRF) grant funded by the Korean Government (NRF2021R1A2C2005820 and NRF-2021M3A9E4021818). Dong Hyeon Lee received the grant from the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (HI21C0538). Sang Min Park received the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT; Grant number: 2021R1F1A1063346) and the SNUH Research Fund (04-2021-0370).
FOOTNOTES
AuthorsŌĆÖ contributions
Conceptualization: SJ, YHO, SC, JC, SMK, JSS, GL, JCA, DHL, BKK, WK, and SMP. Data curation: SJ, SC, JC, SMK, and SMP. Formal analysis: SJ, SC, JC, and SMK. Methodology: SJ, SC, JC, SMK, JCA, DHL, BKK, WK, and SMP. Supervision: WK and SMP. Writing ŌĆō original draft: SJ, YHO, SC, WK, and SMP. Writing ŌĆō review & editing: SJ, YHO, SC, JSS, GL, JCA, DHL, BKK, WK, and SMP.
SUPPLEMENTAL MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
Supplementary┬ĀTable┬Ā1.
Sensitivity analyses on the risk of incident dementia according to the FLI category
Supplementary┬ĀTable┬Ā2.
Multivariate analysis of the key variables on the risk of dementia
Supplementary┬ĀTable┬Ā3.
Multivariate logistic regression of independent significant factors affecting the risk of dementia for propensity score matching
Supplementary┬ĀTable┬Ā4.
Descriptive statistics of the participants with intermediate and low FLI after propensity score matching
Supplementary┬ĀTable┬Ā5.
Descriptive statistics of the participants with intermediate and high FLI after propensity score matching
Supplementary┬ĀTable┬Ā6.
Subgroup analyses on the risk of incident dementia according to the FLI category in the propensity score matching cohort
Supplementary┬ĀTable┬Ā7.
Subgroup analyses on the risk of incident dementia according to the FLI category in the propensity score matching cohort
Figure┬Ā1.
Flow diagram for the inclusion of study population. ALT, alanine aminotransferase; AST, aspartate aminotransferase; ╬│-GT, ╬│-glutamyltransferase; HDL, high-density lipoprotein.
Table┬Ā1.
Descriptive statistics of the participants in the National Health Insurance Service
| Overall participant (n=608,994) | Low FLI (<30; n=193,739) | Intermediate FLI (Ōēź30 and <60; n=145,814) | High FLI (Ōēź60; n=269,441) | |
|---|---|---|---|---|
| Age (years) | 65 (62ŌĆō69) | 65 (62ŌĆō70) | 65 (62ŌĆō69) | 65 (62ŌĆō68) |
| Sex | ||||
| ŌĆāMen | 192,632 (31.6) | 49,541 (25.6) | 42,860 (29.4) | 100,231 (37.2) |
| ŌĆāWomen | 416,362 (68.4) | 144,198 (74.4) | 102,954 (70.6) | 169,210 (62.8) |
| Household income* | ||||
| ŌĆāLower half | 234,675 (38.5) | 74,330 (38.4) | 55,235 (37.9) | 105,110 (39.0) |
| ŌĆāUpper half | 374,319 (61.5) | 119,409 (61.6) | 90,579 (62.1) | 164,331 (61.0) |
| Body mass index (kg/m2) | 23.6 (21.8ŌĆō25.6) | 21.4 (19.9ŌĆō22.8) | 23.4 (22.2ŌĆō24.7) | 25.5 (23.9ŌĆō27.2) |
| Waist circumference (cm) | 81 (76ŌĆō86) | 75 (70ŌĆō79) | 80 (77ŌĆō84) | 86 (82ŌĆō90) |
| Systolic blood pressure (mmHg) | 125 (116ŌĆō135) | 120 (110ŌĆō130) | 125 (116ŌĆō134) | 130 (119ŌĆō138) |
| Diastolic blood pressure (mmHg) | 78 (70ŌĆō80) | 75 (70ŌĆō80) | 78 (70ŌĆō80) | 80 (70ŌĆō83) |
| Total cholesterol (mg/dL) | 203 (179ŌĆō229) | 196 (173ŌĆō220) | 203 (180ŌĆō229) | 209 (184ŌĆō235) |
| Fasting serum glucose (mg/dL) | 95 (87ŌĆō105) | 93 (86ŌĆō100) | 94 (87ŌĆō103) | 97 (89ŌĆō109) |
| Alanine aminotransferase (IU/L) | 19 (15ŌĆō25) | 16 (13ŌĆō20) | 18 (15ŌĆō23) | 23 (18ŌĆō31) |
| Aspartate aminotransferase (IU/L) | 23 (20ŌĆō28) | 22 (19ŌĆō26) | 22 (19ŌĆō26) | 24 (20ŌĆō30) |
| ╬│-glutamyl transpeptidase (IU/L) | 19 (14ŌĆō27) | 14 (11ŌĆō17) | 18 (15ŌĆō22) | 27 (21ŌĆō38) |
| Cigarette smoking | ||||
| ŌĆāNever smoker | 533,111 (87.5) | 172,741 (89.2) | 128,558 (88.2) | 231,812 (86.0) |
| ŌĆāPast smoker | 6,630 (1.1) | 1,755 (0.9) | 1,676 (1.1) | 3,199 (1.2) |
| ŌĆāCurrent smoker | 69,253 (11.4) | 19,243 (9.9) | 15,580 (10.7) | 34,430 (12.8) |
| MVPA | ||||
| ŌĆāŌēż2 times/week | 438,401 (72.0) | 139,442 (72.0) | 103,909 (71.3) | 195,050 (72.4) |
| ŌĆā3ŌĆō4 times/week | 57,730 (9.5) | 18,236 (9.4) | 14,163 (9.7) | 25,331 (9.4) |
| ŌĆāŌēź5 times/week | 112,863 (18.5) | 36,061 (18.6) | 27,742 (19.0) | 49,060 (18.2) |
| HypertensionŌĆĀ | 82,488 (13.5) | 20,370 (10.5) | 19,049 (13.1) | 43,069 (16.0) |
| Type 2 diabetesŌĆĪ | 52,521 (8.6) | 10,758 (5.6) | 11,446 (7.8) | 30,317 (11.3) |
| Dyslipidemia┬¦ | 144,874 (23.8) | 31,155 (16.1) | 33,764 (23.2) | 79,955 (29.7) |
| Charlson comorbidity index | ||||
| ŌĆā0 | 281,453 (46.2) | 96,814 (50.0) | 68,356 (46.9) | 116,283 (43.2) |
| ŌĆā1 | 167,170 (27.5) | 52,580 (27.1) | 40,522 (27.8) | 74,068 (27.5) |
| ŌĆāŌēź2 | 160,371 (26.3) | 44,345 (22.9) | 36,936 (25.3) | 79,090 (29.4) |
Values are presented as median (interquartile range) or number (%).
FLI, fatty liver index; MVPA, moderate-to-vigorous physical activity.
* Proxy for socioeconomic status based on the insurance premium from the National Health Insurance Service.
Table┬Ā2.
Risk of incident dementia according to the FLI category
| Low (FLI <30) | Intermediate (FLI Ōēź30 and <60) | High (FLI Ōēź60) | Ptrend | |
|---|---|---|---|---|
| No. of participants | 193,739 | 145,814 | 269,441 | |
| Overall dementia | ||||
| ŌĆāEvent | 17,512 | 11,741 | 19,285 | |
| ŌĆāPerson-year | 2,057,205 | 1,554,731 | 2,883,416 | |
| ŌĆāaHR (95% CI)* | 0.95 (0.93ŌĆō0.97) | 1.00 (reference) | 1.05 (1.02ŌĆō1.08) | <0.001 |
| ŌĆāaHR (95% CI)ŌĆĀ | 0.96 (0.93ŌĆō0.98) | 1.00 (reference) | 1.04 (1.01ŌĆō1.06) | <0.001 |
| ŌĆāaHR (95% CI)ŌĆĪ | 0.97 (0.94ŌĆō0.99) | 1.00 (reference) | 1.02 (0.99ŌĆō1.05) | <0.001 |
| AlzheimerŌĆÖs disease | ||||
| ŌĆāEvent | 16,984 | 11,335 | 18,533 | |
| ŌĆāPerson-year | 2,064,472 | 1,560,128 | 2,892,412 | |
| ŌĆāaHR (95% CI)* | 0.95 (0.93ŌĆō0.98) | 1.00 (reference) | 1.05 (1.02ŌĆō1.07) | <0.001 |
| ŌĆāaHR (95% CI)ŌĆĀ | 0.96 (0.94ŌĆō0.98) | 1.00 (reference) | 1.03 (1.01ŌĆō1.06) | <0.001 |
| ŌĆāaHR (95% CI)ŌĆĪ | 0.97 (0.95ŌĆō1.00) | 1.00 (reference) | 1.02 (0.99ŌĆō1.04) | 0.004 |
| Vascular dementia | ||||
| ŌĆāEvent | 213 | 188 | 287 | |
| ŌĆāPerson-year | 2,127,747 | 1,601,447 | 2,958,310 | |
| ŌĆāaHR (95% CI)* | 0.80 (0.66ŌĆō0.98) | 1.00 (reference) | 0.90 (0.74ŌĆō1.08) | 0.095 |
| ŌĆāaHR (95% CI)ŌĆĀ | 0.80 (0.66ŌĆō0.98) | 1.00 (reference) | 0.88 (0.73ŌĆō1.06) | 0.098 |
| ŌĆāaHR (95% CI)ŌĆĪ | 0.82 (0.67ŌĆō1.00) | 1.00 (reference) | 0.86 (0.72ŌĆō1.04) | 0.117 |
Table┬Ā3.
Risk of dementia according to FLI groups after propensity score matching
| Low (FLI <30) | Intermediate (FLI Ōēź30 and <60) | High (FLI Ōēź60) | P-value | |||
|---|---|---|---|---|---|---|
| Low-intermediate PSM | ||||||
| ŌĆā | No. of participants | 144,299 | 144,299 | |||
| Overall dementia | ||||||
| ŌĆā | Event | 12,408 | 11,714 | |||
| Person-Year | 1,533,799 | 1,538,099 | ||||
| aHR (95% CI)* | 0.94 (0.91ŌĆō0.97) | 1.00 (reference) | <0.001 | |||
| aHR (95% CI)ŌĆĀ | 0.94 (0.92ŌĆō0.97) | 1.00 (reference) | <0.001 | |||
| aHR (95% CI)ŌĆĪ | 0.96 (0.93ŌĆō0.98) | 1.00 (reference) | 0.002 | |||
| AlzheimerŌĆÖs disease | ||||||
| Event | 12,011 | 11,309 | ||||
| Person-year | 1,539,098 | 1,543,476 | ||||
| aHR (95% CI)* | 0.94 (0.91ŌĆō0.97) | 1.00 (reference) | <0.001 | |||
| aHR (95% CI)ŌĆĀ | 0.95 (0.92ŌĆō0.98) | 1.00 (reference) | <0.001 | |||
| aHR (95% CI)ŌĆĪ | 0.96 (0.93ŌĆō0.99) | 1.00 (reference) | 0.005 | |||
| Vascular dementia | ||||||
| Event | 157 | 188 | ||||
| Person-year | 1,583,546 | 1,584,738 | ||||
| aHR (95% CI)* | 0.81 (0.64ŌĆō1.04) | 1.00 (reference) | 0.100 | |||
| aHR (95% CI)ŌĆĀ | 0.83 (0.65ŌĆō1.06) | 1.00 (reference) | 0.133 | |||
| aHR (95% CI)ŌĆĪ | 0.84 (0.66ŌĆō1.07) | 1.00 (reference) | 0.157 | |||
| High-intermediate PSM | ||||||
| No. of participants | 145,799 | 145,799 | ||||
| Overall dementia | ||||||
| Event | 11,740 | 13,488 | ||||
| Person-year | 1,554,574 | 1,548,333 | ||||
| aHR (95% CI)* | 1.00 (reference) | 1.09 (1.06ŌĆō1.13) | <0.001 | |||
| aHR (95% CI)ŌĆĀ | 1.00 (reference) | 1.08 (1.05ŌĆō1.11) | <0.001 | |||
| aHR (95% CI)ŌĆĪ | 1.00 (reference) | 1.05 (1.02ŌĆō1.08) | 0.001 | |||
| AlzheimerŌĆÖs disease | ||||||
| Event | 11,334 | 12,972 | ||||
| Person-year | 1,559,971 | 1,554,554 | ||||
| aHR (95% CI)* | 1.00 (reference) | 1.09 (1.06ŌĆō1.12) | <0.001 | |||
| aHR (95% CI)ŌĆĀ | 1.00 (reference) | 1.07 (1.04ŌĆō1.10) | <0.001 | |||
| aHR (95% CI)ŌĆĪ | 1.00 (reference) | 1.04 (1.01ŌĆō1.07) | 0.004 | |||
| Vascular dementia | ||||||
| Event | 188 | 202 | ||||
| Person-year | 1,601,279 | 1,600,950 | ||||
| aHR (95% CI)* | 1.00 (reference) | 1.02 (0.81ŌĆō1.27) | 0.889 | |||
| aHR (95% CI)ŌĆĀ | 1.00 (reference) | 0.98 (0.78ŌĆō1.22) | 0.845 | |||
| aHR (95% CI)ŌĆĪ | 1.00 (reference) | 0.94 (0.75ŌĆō1.18) | 0.602 | |||
Abbreviations
aHR
adjusted hazard ratio
A╬▓
amyloid-╬▓
BMI
body mass index
CCI
Charlson comorbidity index
CI
confidence interval
FLI
fatty liver index
FSG
fasting serum glucose
HFD
high-fat diet
ICD-10
International Classification of Diseases tenth revision
IQR
interquartile range
Lcn2
lipocalin-2
LRP-1
lipoprotein receptor-related protein 1
NAFLD
non-alcoholic fatty liver disease
NASH
nonalcoholic steatohepatitis
NFS
NAFLD fibrosis score
NHIS
National Health Insurance Service
NKT
natural killer T
PSM
propensity score matching
REFERENCES
1. Wu YT, Beiser AS, Breteler MMB, Fratiglioni L, Helmer C, Hendrie HC, et al. The changing prevalence and incidence of dementia over time - current evidence. Nat Rev Neurol 2017;13:327-339.



2. Etters L, Goodall D, Harrison BE. Caregiver burden among dementia patient caregivers: a review of the literature. J Am Acad Nurse Pract 2008;20:423-428.


3. GBD 2016 Dementia Collaborators. Global, regional, and national burden of AlzheimerŌĆÖs disease and other dementias, 1990- 2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019;18:88-106.


4. Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med 2018;24:908-922.




5. Satizabal CL, Beiser AS, Chouraki V, Chêne G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham heart study. N Engl J Med 2016;374:523-532.



6. Elwood P, Galante J, Pickering J, Palmer S, Bayer A, Ben-Shlomo Y, et al. Healthy lifestyles reduce the incidence of chronic diseases and dementia: evidence from the Caerphilly cohort study. PLoS One 2013;8:e81877.



7. Bertolotti M, Lonardo A, Mussi C, Baldelli E, Pellegrini E, Ballestri S, et al. Nonalcoholic fatty liver disease and aging: epidemiology to management. World J Gastroenterol 2014;20:14185-14204.



8. Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement 2015;11:718-726.



9. Seo SW, Gottesman RF, Clark JM, Hernaez R, Chang Y, Kim C, et al. Nonalcoholic fatty liver disease is associated with cognitive function in adults. Neurology 2016;86:1136-1142.



10. Solfrizzi V, Scafato E, Custodero C, Loparco F, Ciavarella A, Panza F, et al. Liver fibrosis score, physical frailty, and the risk of dementia in older adults: the Italian longitudinal study on aging. Alzheimers Dement (N Y) 2020;6:e12065.




11. Shang Y, Nasr P, Ekstedt M, Widman L, St├źl P, Hultcrantz R, et al. Non-alcoholic fatty liver disease does not increase dementia risk although histology data might improve risk prediction. JHEP Rep 2020;3:100218.



12. Weinstein G, Davis-Plourde K, Himali JJ, Zelber-Sagi S, Beiser AS, Seshadri S. Non-alcoholic fatty liver disease, liver fibrosis score and cognitive function in middle-aged adults: the Framingham study. Liver Int 2019;39:1713-1721.




13. Labenz C, Kostev K, Kaps L, Galle PR, Schattenberg JM. Incident dementia in elderly patients with nonalcoholic fatty liver disease in Germany. Dig Dis Sci 2021;66:3179-3185.




14. Seong SC, Kim YY, Khang YH, Heon Park J, Kang HJ, Lee H, et al. Data resource profile: the national health information database of the national health insurance service in South Korea. Int J Epidemiol 2017;46:799-800.

15. Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 2006;6:33.




16. European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388-1402.


17. Lee YH, Bang H, Park YM, Bae JC, Lee BW, Kang ES, et al. Nonlaboratory-based self-assessment screening score for nonalcoholic fatty liver disease: development, validation and comparison with other scores. PLoS One 2014;9:e107584.



19. Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol 2004;57:1288-1294.


20. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Metaanalytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73-84.


21. Weinstein G, Zelber-Sagi S, Preis SR, Beiser AS, DeCarli C, Speliotes EK, et al. Association of nonalcoholic fatty liver disease with lower brain volume in healthy middle-aged adults in the Framingham study. JAMA Neurol 2018;75:97-104.



22. de la Monte SM, Tong M. Brain metabolic dysfunction at the core of AlzheimerŌĆÖs disease. Biochem Pharmacol 2014;88:548-559.



23. de la Monte SM. Insulin resistance and neurodegeneration: progress towards the development of new therapeutics for AlzheimerŌĆÖs disease. Drugs 2017;77:47-65.




24. Kim DG, Krenz A, Toussaint LE, Maurer KJ, Robinson SA, Yan A, et al. Non-alcoholic fatty liver disease induces signs of AlzheimerŌĆÖs disease (AD) in wild-type mice and accelerates pathological signs of AD in an AD model. J Neuroinflammation 2016;13:1.




25. Karbalaei R, Allahyari M, Rezaei-Tavirani M, Asadzadeh-Aghdaei H, Zali MR. Protein-protein interaction analysis of Alzheimer`s disease and NAFLD based on systems biology methods unhide common ancestor pathways. Gastroenterol Hepatol Bed Bench 2018;11:27-33.


26. Kanekiyo T, Bu G. The low-density lipoprotein receptor-related protein 1 and amyloid-╬▓ clearance in AlzheimerŌĆÖs disease. Front Aging Neurosci 2014;6:93.



27. Tamaki C, Ohtsuki S, Terasaki T. Insulin facilitates the hepatic clearance of plasma amyloid beta-peptide (1 40) by intracellular translocation of low-density lipoprotein receptor-related protein 1 (LRP-1) to the plasma membrane in hepatocytes. Mol Pharmacol 2007;72:850-855.


28. Sehgal N, Gupta A, Valli RK, Joshi SD, Mills JT, Hamel E, et al. Withania somnifera reverses AlzheimerŌĆÖs disease pathology by enhancing low-density lipoprotein receptor-related protein in liver. Proc Natl Acad Sci U S A 2012;109:3510-3515.



30. Ye D, Yang K, Zang S, Lin Z, Chau HT, Wang Y, et al. Lipocalin-2 mediates non-alcoholic steatohepatitis by promoting neutrophil-macrophage crosstalk via the induction of CXCR2. J Hepatol 2016;65:988-997.


31. Mondal A, Bose D, Saha P, Sarkar S, Seth R, Kimono D, et al. Lipocalin 2 induces neuroinflammation and blood-brain barrier dysfunction through liver-brain axis in murine model of nonalcoholic steatohepatitis. J Neuroinflammation 2020;17:201.




32. Lindestam Arlehamn CS, Garretti F, Sulzer D, Sette A. Roles for the adaptive immune system in ParkinsonŌĆÖs and AlzheimerŌĆÖs diseases. Curr Opin Immunol 2019;59:115-120.



33. Gate D, Saligrama N, Leventhal O, Yang AC, Unger MS, Middeldorp J, et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in AlzheimerŌĆÖs disease. Nature 2020;577:399-404.




34. Grohmann M, Wiede F, Dodd GT, Gurzov EN, Ooi GJ, Butt T, et al. Obesity drives STAT-1-dependent NASH and STAT-3-dependent HCC. Cell 2018;175:1289-1306 e20.



35. Ghazarian M, Revelo XS, N├Ėhr MK, Luck H, Zeng K, Lei H, et al. Type I interferon responses drive intrahepatic T cells to promote metabolic syndrome. Sci Immunol 2017;2:eaai7616.



36. Wolf MJ, Adili A, Piotrowitz K, Abdullah Z, Boege Y, Stemmer K, et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell 2014;26:549-564.


37. Bhattacharjee J, Kirby M, Softic S, Miles L, Salazar-Gonzalez RM, Shivakumar P, et al. Hepatic natural killer T-cell and CD8+ T-cell signatures in mice with nonalcoholic steatohepatitis. Hepatol Commun 2017;1:299-310.




38. Weinstein G, Davis-Plourde KL, Beiser AS, Seshadri S. Author response: non-alcoholic fatty liver disease, liver fibrosis score and cognitive function in middle-aged adults: the Framingham study. Liver Int 2020;40:1240.



- TOOLS
-
METRICS

- ORCID iDs
-
Won Kim

https://orcid.org/0000-0002-2926-1007Sang Min Park

https://orcid.org/0000-0002-7498-4829 - Related articles




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement1
Supplement1 Print
Print



